Design & Engineering
Graphic featuring MD+DI Senior Editor Amanda Pedersen for her weekly medtech opinion column, Pedersen's POV.
Design & Engineering
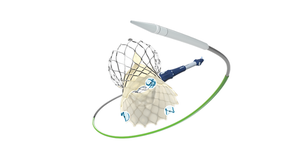
Cool Device, Bruh, But Does It Work?Cool Device, Bruh, But Does It Work?
This week in Pedersen's POV, our senior editor weighs in on an evolving debate in medical device design.
Sign up for the QMED & MD+DI Daily newsletter.






.png?width=300&auto=webp&quality=80&disable=upscale)




.png?width=300&auto=webp&quality=80&disable=upscale)


![Featured Image] 230916SF73.jpg Featured Image] 230916SF73.jpg](https://eu-images.contentstack.com/v3/assets/blt14ac89070d5e4751/blt381af30a9066bd0b/655b99d3323a8f040a9ee156/Featured_Image_230916SF73.jpg?width=300&auto=webp&quality=80&disable=upscale)