October 21, 2015
Imagine you are working beneath a car and the car jack suddenly fails. Expandable (or telescopic) spinal implants for anterior cervical corpectomy can be compared to a car jack--when they collapse in the cervical spine, the consequences can be grave, including neurocompromise, paralysis, and respiratory arrest.
Qmed Staff
|
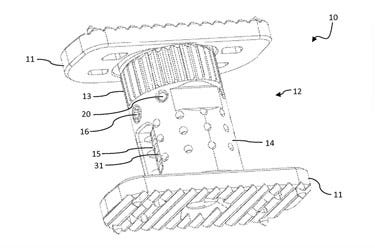
A patent drawing for the X-Core |
NuVasive recently announced that it has received the first 510(k) clearance for the X-Core Mini Cervical Corpectomy System, an expandable titanium vertebral body-replacement device, which, similar to a car jack, is a relatively small device that supports the weight of an object much heavier than it itself is.
The company says the 510(k) is a "first-of-its kind clearance" that "showcases NuVasive's commitment to investing in innovative technology and clinical research to improve patient outcomes."
The device itself, however, is not new and its predicate has been linked to at least 15 adverse events. According to the K151651 file, the 510(k) application seeks to expand the indications for use of the predicate device, the X-Core Expandable VBR System, "to include treatment of tumors, trauma, and degenerative disorders of the cervical spine." The FDA clearance summary for the new X-Core Mini goes on to state, "no other changes have been made to the system design since its clearance in the [predicate] X-Core Expandable VBR System K142205. Therefore, no new nonclinical testing was performed for the purpose of this submission."
NuVasive states that the predicate device, X-Core VBR, "has a safety and effectiveness profile" similar to the newly cleared X-Core Mini. The X-Core VBR, however, has been linked to the adverse events shown in Table 1. Of those events, the vast majority of which were used below the neck. Yet cervical corpectomy is a uniquely risky procedure. A 2002 study reported that for 40 procedures who underwent the procedure, nearly half (47.5%) had perioperative complications.
TABLE 1. ADVERSE EVENTS ATTRIBUTED TO X-CORE
DATE | DEVICE | PROCEDURE | EVENT | REVISION | MDR KEY |
3/25/15 | X-Core | L1 Corpectomy | 5mm height loss | Not planned | |
2/4/15 | X-Core | L3 Corpectomy | Device came apart | Yes | |
1/14/15 | X-Core | L3,4 Corpectomy | Loss of height | Yes | |
12/2/14 | X-Core | L2,3 Corpectomy | Loss of height | Not reported | |
2/26/14 | X-Core 2 | T12-L1 Corpectomy | Loss of height | Yes | |
12/23/13 | X-Core 2 | L2 Corpectomy | Loss of height | Yes | |
4/1/13 | Reported as Adjustable VBR (but part no. 5962366 belongs to X-Core Mini) | C3 corpectomy | device subsided into C3 body six weeks after surgery | Yes | |
2/12/2013 | X-Core | T12 Corpectomy | Transverse Plate Backed out | Yes | |
1/14/2013 | X-Core | T8-10 Corpectomy | Loss of height | Yes | |
6/6/2012 | X-Core | Not reported | Loss of height | Not reported | |
3/20/2012 | X-Core | Not reported | Partial collapse | "No plans" | |
2/7/12 | X-Core | T11-L1 Corpectomy | 4-5mm collapse | "Not planned" | |
5/4/2011 | X-Core | Unknown | "Reduced height" | Unknown | |
4/1/2011 | X-Core | L1 Corpectomy | 1-1.5 cm height loss | Not "yet" | |
4/1/2011 | X-Core | T11 | Height loss | Yes |
Though Qmed previously reported seven adverse events associated with the X-Core, eight additional adverse events were found upon further review of the FDA MAUDE database, including a 2013 report of a device with part number 5962366, which corresponds to NuVasive's recently cleared X-Core Mini, which was not cleared until just this past September 2015. The adverse event report stated that the device, described as the "Adjustable VBR," had subsided into the cervical spine, injuring a patient. Subsidence occurs when the device pistons through the bottom of the vertebral body pushing like a nail through soft bone. The event, like the seven other adverse event reports, required reoperation.
When using FDA's total product life cycle (TLPC) database, only 5 adverse events show attributed to corpectomy device "collapse" -- four of them attributed to the X-Core.
Part of the reason for corpectomy's unique risk is the unique biomechanical profile of the cervical spine compared to the thoracolumbar spine. In addition,the anatomical proximity of an implanted cervical corpectomy device in the neck would lead to greater risk of injury to nerves that control the hands, arms, and the diaphragm which controls breathing. Even though the FDA had never approved or cleared a device for cervical corpectomy before, NuVasive was able to submit its expanded use of the previously cleared X Core K142205 under the 510(k) rather than premarket approval (PMA) process which is reserved for first-of-its-kind, novel medical devices.
Before FDA cleared the X-Core Mini, only one other device, had FDA permission to be marketed for cervical corpectomy. The Telescopic Plate Spacer (TPS-C) (Biomet Spine, Broomfield, CO) VBR system received a Humanitarian Device Exemption (HDE) approval from the FDA for reconstruction of C3-T2 following surgery for tumors. A search of FDA's MAUDE database, which houses the biometric data on reported adverse events associated with medical devices, lists "no records found" on the TPS-C, though it was withdrawn from the market after a study showed 43% subsidence and 20% reoperation rates.
As alluded to in the beginning of this article, the design construct of expandable corpectomy devices are similar to a hydraulic tire jack. Once a void is created by the corpectomy, which involves removing a vertebral body and adjacents discs, the device is inserted into the space and distracted (expanded) like a tire jack to achieve the desired height. Bone graft material is packed inside the device to promote fusion between the upper and lower vertebra. However, if collapse or loss of height occurs, neurocompromise can result . The X-Core shows that seven of the 14 reported adverse events reported by NuVasive involved device collapse requiring reoperation with no reports of injury, only device malfunction.
The Synex (Synthes) corpectomy device, also FDA cleared for use only in the thoracolumbar spine, was cited by NuVasive as a predicate device for its X-Core device. After just six adverse events reporting similar loss of height, FDA issued its most urgent class I recall out of concerns that "there is a reasonable probability that the use of, or exposure to, a violative product will cause serious adverse health consequences or death."
Nerves that control the hands, arms, legs, bladder, and diaphragm and airway are in close proximity of the cervical spine in the neck. Any injury from device collapse as those listed for the X-Core or Synex in the cervical spine could result in life-threatening or life-altering complications such as paralysis, quadriplegia and respiratory arrest.
NuVasive did not immediately respond to a request for comment.
Learn more about cutting-edge medical devices at Minnesota Medtech Week, November 4-5 in Minneapolis. |
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like