March 15, 2016
FDA has given Class I status to a field safety notice for Abbott's MitraClip delivery system.
Qmed Staff
 [Updated March 17]
[Updated March 17]
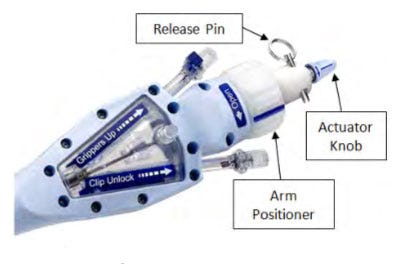
On February 15, FDA announced that Abbott Vascular has received nine reports of its MitraClip delivery system failing to detach from the Clip, which resulted in the death of one patient and open-heart surgery to extract the device. The problem can also cause its mandrel component to fracture. "Tension is present if the Arm Positioner is on the 'Closed' side of Neutral, as opposed to being in the Neutral position during Clip deployment," reads the company's field safety notice.
Abbott's response to the problem was to provide updated instructions and training for clinicians using the device--touted as the world's first percutaneous mitral valve repair system. "I wanted to clarify that Abbott did not recall any product," says Jonathon Hamilton of Abbott Public Affairs. "All products in the market are safe if used as directed in the instructions for use." The company is also offering to train users of the device to reinforce the proper procedures used to operate and deploy the device, Hamilton says.
Abbott identified that the delivery system's arm positioner was in the wrong position and, therefore, did not return to the neutral position during use, thus preventing the clip from disengaging.
The company noted that this problem only occurred in 0.17% of cases where the MitraClip was used.
Abbot explains that it is revisiting its current instructions for use to avoid the problem. While the current instructions specify that arm positioner is in a neutral position before turning the actuator knob to deploy the MitraClip, the new instructions will "provide additional assurance that tension is completely eliminated prior to deploying the Clip," reads the field safety correction.
Jason Rogers, MD of the University of California Davis Medical Center was quoted by MedPage Today as stating that the problem could be "rectified through a simple modification in how we release the clip," thus eliminating "any chance of malfunction."
There were 3534 devices on the market--including 1288 in the United States. Intended to treat mitral regurgitation, the device is indicated for use in patients for whom mitral valve surgery is contraindicated.
The company has determined that products with lot numbers of 50714U1 and higher have the problem.
In related news, a registry analysis concerning the MitraClip found that it successfully treated roughly 91% of patients with primary mitral regurgitation. In the study, success was defined as a reduction of slight to moderate mitral regurgitation without cardiac surgery.
The study was led by Paul Sorajja, MD from the Minneapolis Heart Institute at Abbott Northwestern Hospital and was published online in the Journal of the American College of Cardiology.
Roughly 600,000 people in the United States suffer from mitral regurgitation while a substantial number of those patients are not candidates for surgery.
The MitraClip was approved by FDA in October 2013.
Note: An earlier version of this article incorrectly stated that the FDA Class I recall for the MitraClip component related to an actual recall. In fact, Abbott did not recall the device but only released a field safety notice for the device.
Learn more about cutting-edge medical devices at BIOMEDevice Boston, April 13-14, 2016. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
About the Author(s)
You May Also Like


