Sunshine Heart halts its pivotal trial to test a novel heart assist device meant to be an alternative to left ventricular assist devices because of patient deaths.
March 9, 2015
By Arundhati Parmar
|
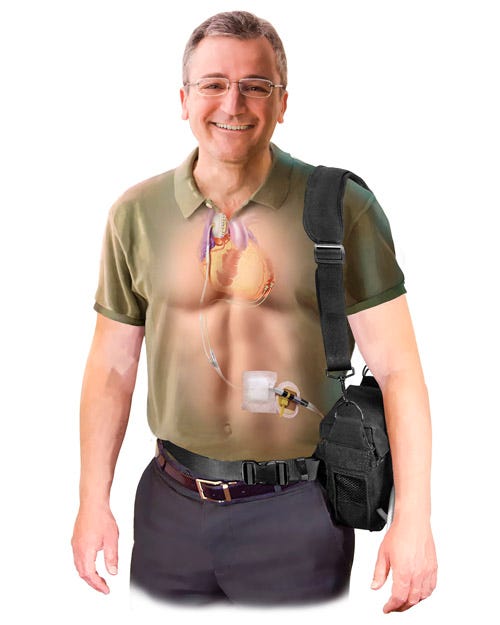
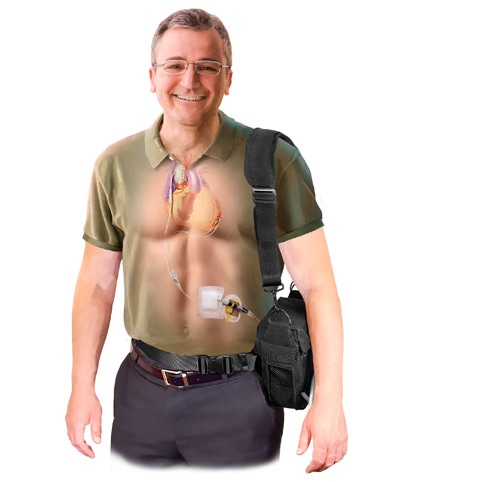
The C-Pulse Heart Assist System |
Sunshine Heart has been at it for years to provide a safer, more effective alternative to left ventricular assist devices for heart failure patients.
On Friday, the Minnesota company announced that it was halting enrollment in its pivotal trial due to four patient deaths. Two of the deaths have already been ruled unrelated to its C-pulse heart assist system, a non-blood contacting, implantable device aimed at patients with moderate to severe heart failure. And the company believes that the other two deaths are also not related to the device.
But the news release points out that the study has to be halted per its protocols when more than three of the first 20 trial subjects pass away for any reason.
The FDA has asked the company to submit an IDE supplement that will detail why the study was halted and how Sunshine Heart will resume enrollment. That is expected to be filed by March 16.
"We are confident this matter will be resolved in a very short timeframe," said Dave Rosa, Sunshine Heart's chief executive officer. "While the current data suggest these incidents are non-device related, we have decided that in the absolute interest of patient safety, having a temporary pause in enrollment is the right course of action while we work with the FDA to discuss the findings."
About a year ago, Rosa explained to MD+DI how the novel device has the potential to cure heart failure in some patients.
Nonetheless, the company has had to deal with a slow enrollment of the pivotal trial. The company has about 100 patients enrolled while the Counter HF trial is expected to enroll 388 patients in up to 40 clinical sites.
Meanwhile when it comes to heart assist devices, looks like pivotal trials may be jinxed.
A report from EP Vantage, the publishing arm of Evaluate Group, a London-based life science market intelligence firm, points out that the suspension of the C-Pulse device follows the halt of the the REVIVE-IT trial of Thoratec that is studying the company's Heart Mate II device. The report notes that the pause in enrolling patients for the trial is not related to safety concerns,
You May Also Like