The rapid and successful completion of clinical trials is a prerequisite for the commercial success of any product. Early adoption of an iterative design process is vital to this success as the approach insists that both the environment in which the product will be used and the end user is considered from the outset. The process also encourages a practical view to be taken on the balance that needs to be struck between engineering functionality for clinical trial and design and the management of specification creep.
The rapid and successful completion of clinical trials is a prerequisite for the commercial success of any product. Early adoption of an iterative design process is vital to this success as the approach insists that both the environment in which the product will be used and the end user is considered from the outset. The process also encourages a practical view to be taken on the balance that needs to be struck between engineering functionality for clinical trial and design and the management of specification creep.
Where product development is considered for industrial applications beyond medicine and healthcare, a prototype is often defined as a first or preliminary version of a device from which other forms are developed. Development houses operating in the commercial world will usually follow a design process that enables them to move concepts swiftly from prototype to launch. However, while the actual design process and milestones for medical and non-medical sectors is remarkably similar, product developers in the medical and life-science sectors can’t move so freely. They need to work around the barriers and limitations posed by the regulatory requirement to prove function and safety through appropriate clinical trials.
It’s true that prototype manufacturing techniques can produce products or devices suitable for clinical trials. But defining these units as prototypes in the purest sense of the term can be misleading.
Pilot Products, Not Prototypes
Often, there is a strong drive for emerging medical technology companies to move devices into clinical trials as quickly as possible. This represents a key and often fundamental milestone, not just in the product development cycle, but also in the development of the business itself. For many companies, achieving a clinical proof-of-principle often represents the basis on which follow-on funding or business exit strategies depend. Hence, to bring products closer to the market while adding value to their business, organizations—particularly those backed by venture capital—are driven to seek the fastest route to the production of devices suitable for clinical trials.
Considering the products that are built for these trials as prototypes can send the wrong messages, both to the regulator and to potential investors. It suggests the that design might be provisional, or early, and might be subject to change. In fact, however, the opposite is often true. In the consumer products industry, for example, developers often create a limited number of devices for the purposes of technology demonstration or for marketing support.
This type of prototype is usually produced in low numbers, using specialist manufacturing methods. Although costly, it represents the most rapid route to a “looks-like,” “works-like” demonstrator that’s perfectly acceptable for this industry sector. Outside of the medical and life-science industries, market acceptance of these demonstrators is suitable justification to proceed with the capital investment in production tooling, manufacturing facilities, and ultimately product launch.
It happens this way for two main reasons. First, these industries are often led by market trends and, therefore, the need to get products rapidly onto shelves is paramount to their success. Second, the regulatory requirements are generally less stringent. The need to prove the product’s robustness prior to launch through statistical analysis is reduced.
This approach doesn’t always fit with the medical device industry’s requirements where prototypes are required in larger numbers prior to launch to prove the statistical analysis of results gained from clinical trials that the device fulfils its specified requirements. At this stage, rather than call a device a prototype, it would be described better as a pilot manufacture product.
Toy with a comparison
By way of illustration, consider the typical design steps that a toy manufacturing company might take when developing a new action figure. Interestingly, the steps for developing a toy would initially follow a path that’s similar to that of a medical device.
The toy will have begun life as a concept idea or sketch. It would also have been through a review and approval process along with a selection of alternative designs that might be considered for further development. It’s likely that some level of early risk analysis will have taken place and some provisional changes to the design intent made. At this stage, the design lead responsible for the concept likely will have considered not only how the product should look aesthetically, but also how it should perform. He also will have considered how it might be manufactured. These are the same processes that the development of almost any medical device would take.
The next steps would be to prototype the selected concepts rapidly. At this stage, the toy company would expect to invest in the procurement of a small number of high-value, one-off, prototypes. These would typically be handmade by a specialist model-maker, using rapid techniques to ensure that the final prototype is as close as possible in form and function to the final intended production versions.
It’s highly likely that these prototypes would be utilized within the toy company for marketing and promotional purposes. It will also be used by the in-house development team and by production experts as a benchmark for any further mechanical development.
The regulatory barrier
The next phase of design involves the need to statically prove the product’s functions, robustness, or intended manufacturing methods would be reduced. In fact, it’s unlikely that these prototypes will be used for anything other than marketing or early user handling studies and in a highly supervised environment. Proof-of-principal testing would occur at some level to verify the product’s functions against the specification requirements. It’s unlikely, though, that this will be backed up by product trial or field testing in large numbers prior to launch.
There are, of course, some clearly defined conformance requirements, such as the CE mark certification, which crosses both the medical and commercial fields for EU products. However, the driving requirement in most non-medical industries is to ensure that the product complies with all the essential safety requirements prior to launch. Then the manufacturer must act swiftly on feedback from a strong customer quality-assurance feed-back process. This enables design revisions to incorporate feedback from consumers and users resulting from defects and failures observed in the field.
In a somewhat different manner, regulation within the medical sector demands that a prototype used for this level of evaluation be identical to a final release product. Hence, there’s a preference to call it a pilot-manufacture product, rather than a prototype. At this stage, a change-control process must be in place along with detailed records of any prior testing or development work that might influence the product’s performance.
This data is eventually compiled as a critical part of the design history file (DHF), a vital component of a regulatory approval submission. Confidence in the design robustness and flexibility must be proved and recorded, while the quality process must be adhered to and withstand regular audits. All of these elements must be in place, and proven, prior to any regulatory approval for product resale.
Continuing with the toy example, acceptance of the prototype from both the marketing and design teams would normally be adequate validation for the company to invest in further development. Their aim would be to move swiftly toward preparation and procurement of the necessary production tooling. Regulatory and quality assurance testing of this new action figure will mostly take place using initial production products, and any safety modification can typically be implemented rapidly under a change-control procedure, thus enabling the product to be deemed safe for commercial resale in a matter of weeks.
For commercial products, two factors drive this rapid cycle from prototype to production. First, the regulatory requirement that ensures the product is robust and functionally safe. Unlike medical products, toys aren’t critical for maintenance of life. The risks associated with playing with the toy are low compared to the function of many medical devices. Another influencing factor, lower on the risk scale but important for the development of commercial products, is the expected lifecycle. A toy is generally intended to have a two- to three-year shelf-life. A change in market trend or fashions will demote the product swiftly to the bottom of the toy box. Non-medical industries know this and move continually onto the next product while the designers of medical products have to consider a longer future for their designs.
Understand the requirements
It’s clear that when embarking upon a medical-device development program, a sound understanding of which regulatory requirements apply to that specific device and, in turn, what the regulatory obligations are likely to be, is of paramount importance. The complexity of these requirements—and the need to navigate through and around them—are what make the medical development territory so different from other sectors.
The appointment at an early stage of a regulatory advisor to shed light on regulatory compliance can significantly impact the shape of the development program. While there is some variation in the regulatory requirements between different authorities (with US regulatory compliance often being perceived by developers as being more stringent than other countries), certain key device considerations must be demonstrated through clinical trials.
Successful trials depend on some important questions being answered. Among them are, how do you make a device safe? How do you ensure the device operates always as users expect? And how do you know what user’s expectations are?
Put the user first
As with product development in most commercial industries, at the earliest possibility, often at the concept generation stage, thought should be given to the intended users of a device and the situations in which it will they will use it. It’s not unusual for medical device developers to be focused almost exclusively on achieving a technical proof-of-principle without the user-related aspects of the device being properly considered. This lack of thought might result from funding limitations or simply that the value placed on this part of the design aren’t high enough at the early stages of development. While there might be an intention to revisit these design considerations later, delaying their evaluation just defers usability and design problems to a time when it can be more costly to modify the device.
Interaction design
An “interaction design” approach can result in creative tension between various functional groups (see Figure 1). However, it’s a vital element to integrated product development. It should also be noted that the level of detail of interaction design should be appropriate to the specific project, and that it should be undertaken with a view to it becoming part of the device specification.
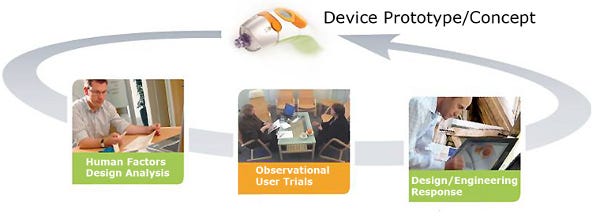
1. Interaction design principles.
It’s important that throughout the concept generation and into the proof-of-principle, the views of stakeholders, beyond the design and engineering team, is included to inform each design response and iteration loop. Among these stakeholders should be relevant healthcare professionals and patient representatives.
Design control, which is essential for maintaining a design history file for the purposes of regulatory submission and approval, starts with the user requirements specification (URS). The URS should be a succinct document that describes what the product must be at a level that’s easily understood by any reader.
The URS first comprises the objective(s), goals, and functions of the required product and doesn’t normally have precise limits. Second, it describes the product aims, which should be as specific as possible, without indicating solutions or limiting the scope of possible solutions. And third, is what’s not yet known or is still unclear, within the context of the product overview. This is a point at which many projects fail, in that they don’t correctly specify what the system should do.
Once the URS has been written, a product requirement specification (PRS) should be drafted. The PRS is a more detailed document, written to define the characteristics for the product concerned. This could include performance, functional parameters, cost, and production volumes.
Requirements must be stated clearly, covering “must” and “want” metrics or attributes. A means of verification for any characteristics must also be included, and this forms the basis of a design verification plan (DVeP). Verification activities often form the basis of the design iteration loop.
An example of a developmental process for medical device development is shown in Figure 2. Defining and achieving clear development milestones is important in transitioning from one development phase to the next, and then through to industrialisation of the product.
.jpg?width=700&auto=webp&quality=80&disable=upscale)
2. A development process for medical device development.
It’s important to remember that while the PRS will capture all the requirements for a product, it might not be practical or necessary to incorporate all these features for the purposes of clinical trials. And it may be that device development programs that choose to carry forward all the features detailed within the PRS become financially burdensome.
For example, in the case of an in-vitro diagnostic product, the primary endpoints relate to demonstrating safety and the effectiveness of the assay. In this case, all target product profile considerations should be prioritized, such as incorporation of a printer or docking station, or communication with a Laboratory Information Management System (LIMS).
These product requirements may be added to the device as part of a post-marketing authorization modification. Additional, but limited, clinical trials will be required, but the focus will be on being able to demonstrate that there’s no change to the system’s performance in terms of safety and effectiveness.
Rapid prototyping
The use of rapid prototyping resources, now commonly available, enables device concepts to be evaluated and iterated by designers in days rather than months. This enables accelerated iteration cycles to be completed prior to the design verification and early entry into certain trials, such as user validation studies. The prototyping techniques and methods available will be entirely dependent on the type of product that’s being developed. For example, consider the development of a new point-of-care diagnostic reader, a product that’s intended to be located in a central location within small GP clinics. It comprises a reader unit and disposable single-use cartridges. The reader unit, which is a high-value, low-volume production product, uses low-value, high-volume, disposable cartridges for the samples. The methods of approaching prototyping production for a clinical trial for both of these aspects of the reader will be different.
From designs to products
Following the design process from the initial concept selection, nominated designs can be worked-up rapidly in silico using a combination of 3D computer-aided design (CAD). Software is available to enable both the electro-mechanical designers to work on the electronic software and hardware for the diagnostic reader, and the product designers to develop the physical aspects, such as the caseworks, keypads, and disposable cartridge enclosures. The design team would aim to move swiftly to low-level, semi-functional prototypes using rapid 3D printing techniques such as stereolithograpy (SLA) and early reprogrammable PCBs to prove the concept.
It’s also possible to evaluate and select the final materials from which the product will be manufactured. The significance of this is that the expected performance, robustness, and ultimately, the cost of the device can be determined early in the development process.
For the disposable cartridge element, it’s likely that several thousand may be required to yield sufficient numbers for a successful clinical trial. Therefore, generating rapid prototype injection moulding tooling and suitable assembly and processing equipment to enable low-volume production of components that are representative of the final design will be required. In contrast, the diagnostic reader unit may only be produced in low numbers for the trials. Hence, different techniques can be used for its production. For the electronic hardware elements, it’s likely that the designers will choose to use production methods similar to the final intent to aid eventual validation, but this choice will come down to the costs and time available.
The tooling used for the production of clinical trial devices is likely to differ from the ultimate production variants as they are likely to be far simpler, producing single parts for hand assembly as opposed to multiple batches of parts for fully-automated assembly. Prototype tooling would be inefficient for production, but it enables prototypes to be produced rapidly for trials. It’s worth noting that good quality prototype tooling can also be capable of producing many thousands of parts and is often sufficient for initial production launch volumes.
The development challenges faced by medical product developers producing these pilot production products will be recognizable to those working on products in other sectors. However, the need to produce early production models for clinical trials that are indistinguishable from a final product and within a regulatory landscape that’s far more complex than in many sectors is what makes it so different from the non-medical world. Also, the users of medical products are more diverse than the customers of many sectors. Understanding their needs is critical to success.
Producing accurate pilot products and completing clinical trials successful is imperative and there’s no other way to support the development, verification, and manufacture of medical products other than to implement well-designed and adequately controlled clinical trials. The commercial risk associated with market access can be reduced significantly by taking a genuinely integrated approach to device design and prototyping.
Real-world example
Team Consulting assisted a Dutch biotech firm, ProFibrix, in the development of a new wound-sealing product. Called Fibrocaps, it’s a dry powder fibrin sealant, based on a mixture of the proteins fibrinogen and thrombin which occur naturally in blood. When applied to a wound Fibrocaps precipitates clotting and stops bleeding that occurs during surgery or after injury. Unlike existing liquid Fibrin sealant sprays, which need to be refrigerated and mixed directly before use, Fibrocaps are prepared in seconds and can be stored at room temperature.
However, applying a powder, which comprises fine particles that become sticky when moist, to bleeding sites presents many technical challenges. ProFibrix conceived a method of application by which a hand-held device ‘blows’ the sterile product onto the wound. The company sought a partner to define a technical design and produce prototypes that would enable ProFibrix to move to phase II clinical trials. Team Consulting was selected to develop a prototype device, compliant with ISO13485, the quality standard which governs the design and manufacture of medical devices.
Team Consulting took conceptual ideas and, in conjunction with clinical users, developed them through a series of product definition and technical workshops. User feedback enabled design refinements and, following rigorous testing and development, the project team arrived at a proof of principle design. In about four weeks, Team Consulting had procured the automatic injection mould tooling needed to produce components for the device, enabling the rapid manufacture of disposable prototypes. ProFibrix has now entered Phase II clinical trials using the device.
Dr. James Blakemore is a molecular biologist by training with a BSc (Hons) from Edinburgh University. As a senior consultant at Team Consulting, he leads in the area of in vitro diagnostics. Brennan Miles is a product design consultant for Team Consulting. He holds a BSc (Hons) in Product Design and has ten years experience in product development.
About the Author(s)
You May Also Like

