February 5, 2016
3-D printing technology is a perfect fit for creating head implants that are customized to a user. Recently, the company BioArchitects announced that it had won 510(k) clearance for its patient-specific titanium cranial/craniofacial plate device.
Brian Buntz
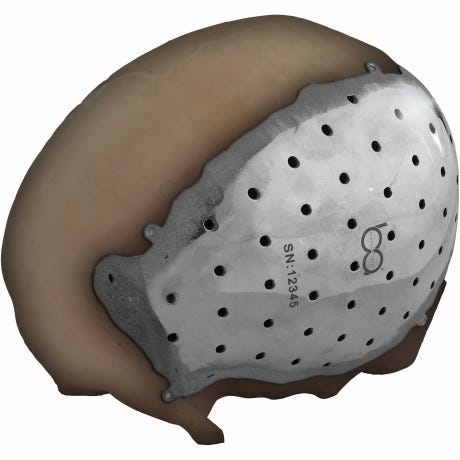
A cranial/craniofacial device from BioArchitects is intended to be used to repair defects in non-load-bearing bones in the head and faced. The implant is affixed to the skull or face using self-tapping titanium screws.
The company says that the devices leverages 3-D printing technology from Arcam AB, which is used to shape a lightweight titanium alloy with high tensile strength that makes use of Arcam's proprietary Electron Beam Melting technology.
The device is created by using data gathered from either a CT or MRI scan of the region in question. The scan is then imported into a computer design program that is then used to make a template for the plate to repair the defect. A surgeon and biomedical engineer collaborate on the design of the final product. The finished product looks something like a jigsaw puzzle piece.
Getting from CT scan or MRI, the finished plate is the end-product of collaboration between the surgeon and the biomedical engineer responsible for the design.
"We are extremely proud to contribute to what we consider another major advance in the trend toward personalized medicine. We believe that this is yet another step toward what will ultimately become the new standard of care," said Mark Ulrich, CEO of BioArchitects USA in a press release.
In 2014, a Dutch woman received a 3-D printed skull.
Learn more about cutting-edge medical devices at MD&M West, February 9-11 at the Anaheim Convention Center in Anaheim, CA. |
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like


