3D Printing
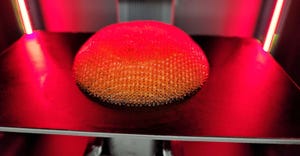
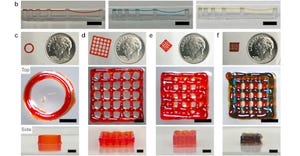
Formlabs' Form 4 3D printer
3D Printing
Formlabs Releases Fastest 3D Printer YetFormlabs Releases Fastest 3D Printer Yet
The Form 4 can achieve vertical print speeds of 100 mm per hour and outpace injection molding, according to the company.
Sign up for the QMED & MD+DI Daily newsletter.


.png?width=700&auto=webp&quality=80&disable=upscale)