May 22, 2014
3-D printing is on everyone's lips. But is it ready for prime time? Are existing materials and processes ripe enough to replace such tried and true manufacturing methods as CNC machining and injection molding? While it can serve as a rapid prototyping technique, can it make the leap to full-on manufacturing? Andres Bernal, founder of Bioniko Consulting LLC (Sunny Isles, FL) will take a stab at these and other questions on Wednesday, June 11, at MD&M East in a presentation titled "Leveraging 3-D Printing for Medical Device Design and Manufacturing." In the meantime, enjoy the following Q&A with Bernal.
MPMN: How is 3-D printing being used in the medical device industry to create prototypes?
|
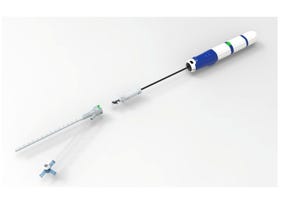
A syringe-handling mechanism consists of two 3-D printed parts and a turned aluminum shaft. This assembly consolidation went from concept to functional prototype in three weeks. |
Bernal: The medical device industry has been using 3-D printing for prototyping applications because this technology offers a range of advantages over conventional manufacturing methods. One key advantage is the concept of a 'build'. A build can be defined as the end result of the 3-D printing process. As opposed to machining, casting, or molding processes, which usually produce single parts or multiple copies of a single part, a 3-D build can be made up of one part, several copies of that part, different iterations of that part, a variety of different parts, and even preassembled parts...or all of the above at once. This capability gives designers the ability to iterate in parallel, reduce assembly steps and ultimately increase prototyping throughput, greatly accelerating the development process.
Along with the aerospace industry, the medical device industry is among the biggest users of 3-D printing. Both industries have capitalized on the ability to add geometrical complexity to their designs without adding significant cost and man-hours, in contrast to using conventional manufacturing methods. This is useful not only in terms of innovative device design but also in opening the door to geometries based on human anatomy. If I had to name a key contribution that 3-D printing has made to the medical device industry, I'd emphasize its ability to print anatomies and anatomically derived parts. 3-D printing not only provides the ability to fabricate patient-specific instruments and implants but also anatomical replicas of body parts such as the heart. It can also produce intricate bony structures such as those seen in craniofacial surgery cases or even color-coded models of the brain for surgical planning. Anatomical structures have nondeterministic geometries, which are really difficult to recreate using molding, CNC machining, or any other type of conventional manufacturing technique.
MPMN: What challenges does 3-D printing face in producing medical device prototypes?
Bernal: While the selection of 3-D printing materials is still limited, they are evolving quickly. It is possible to group these materials into three different categories. The first is the photopolymer family, which includes stereolithography (SLA) materials and those used in PolyJet printers. These technologies use mostly proprietary materials, but some have passed USP class VI testing. The second category includes powders used in sintering machines. These machines can make parts from such metals as stainless steel, titanium, and chromium cobalt and also out of medical-grade polymers such as PEEK. The third category includes materials employed in fused deposition modeling, the most economical 3-D printing method. These materials include real thermoplastics such as ABS and such medical-grade materials as ULTEM. Noticing the growing prevalence of this technology in the medical device sphere, 3-D printing manufacturers are trying to develop material solutions to accommodate this trend.
A major challenge facing 3-D printing of prototypes is the regulatory dimension. Because some medical devices are subject to toxicity, biocompatibility, and sterilization requirements, they must be prototyped and tested as production-equivalent parts in the final materials. Most of the time, however, 3-D printed prototypes are not production equivalents. Whereas some high-end 3-D printing technologies such as selective laser sintering, electron beam melting, and direct metal laser sintering can process end-use materials, these technologies are not generally intended for use in mass manufacturing. Thus, at some point, testing will have to be conducted on prototypes made using conventional methods.
This challenge will gradually be overcome as 3-D printing is used as the final production method. This is happening more and more. In short, 3-D printing manufacturers and materials suppliers serving the medical device industry have realized that there is a need to bridge the gap between prototyping and production. Thus, there's a big push to develop USP-certified materials, some of which are already on the market. And for sintering machines, such materials as titanium, chromium cobalt, and PEEK are being used to produce end-use implantable devices. Thus, prototypes are becoming end-use parts.
MPMN: What do you see as the future of 3-D printing of medical device prototypes?
Bernal: The future of medical 3-D printing lies in blurring the line between prototypes and end-use parts. In conventional manufacturing methods, there is usually a defined 'design transfer' step between prototyping and manufacturing, during which the manufacturer has to translate the design intent of the final prototype into a manufacturing process. This involves design for manufacturability analysis; many technical drawings; and, in most cases, tooling design and manufacturing. This is the point of no return, because once tooling has been made, any change to the design will be very expensive. In the future, prototypes made using 3-D printing will be indistinguishable from end-use parts in form and material. Hence, the design transfer process and cost will be dramatically reduced for some medical device applications. This will translate into an accelerated product cycle in which improvements can be prototyped and commercialized almost immediately.
Aside from closing the gap between prototyping and production, the future course of 3-D printing will involve new materials, higher equipment speeds, more machine capabilities, bigger platforms, and parts with finer details. However, 3-D printing is not magic; it's not as if you insert a CAD file into the system and you're ready to roll. The 3-D printing process requires a considerable amount of postprocessing and part finishing steps to remove support structures. At present, these steps are somewhat labor intensive. Chemicals need to be added, sanding is required, and other types of finishing processes are needed to make 3-D printed parts suitable for use. In the future, postprocessing steps will hopefully be more automated and streamlined.
3-D printing can currently manufacture synthetic models of anatomical structures for use in surgical planning, device testing, or medical education. But there is increasing research to replace synthetic raw materials with organic materials and living cells in order to create functional organs. This capability could be a reality within the next decade.
Bob Michaels is senior technical editor at UBM Canon.
About the Author(s)
You May Also Like