April 24, 2015
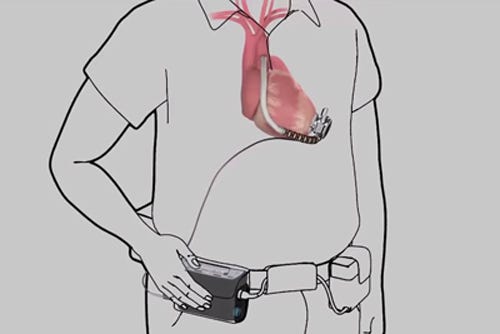
1. Insufficient TestingGoing about electrostatic discharge testing (ESD) in the wrong way can be perilous, as a popular YouTube video titled “Don't worry, it's just ESD!” points out. Not doing any kind of requisite testing for that matter can be problematic, of course.Evidently, HeartWare (Framingham, MA) made that mistake when developing controllers for a clinical trial of its implantable heart pumps. The older controllers, used by about 120 U.S. patients, exhibit a higher susceptibility to electrostatic discharge (ESD) than the company’s newer, commercial controllers.Such an ESD could cause the pump to stop, leading to serious injury or death, the company said. Since a voluntary Field Safety Corrective Action in 2013, HeartWare has received reports of one additional death and one additional serious injury in which ESD may have caused or contributed to a pump stop.Continue >>
10 Causes of Medical Device Failure
April 2015
When medical devices fail, the consequences can be tragic. Here are 10 common reasons they do.
Learn more about cutting-edge medical devices at MD&M East, June 14–15, 2016 in New York City. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz. Chris Newmarker is senior editor of MPMN and Qmed. Follow him on Twitter at @newmarker
Like what you’re reading? Subscribe to our daily e-newsletter.
Image above inspired by figure from an FDA guidance document.
You May Also Like


