Medtronic’s Newest Generation Evolut FX+ System Nabs FDA Nod
The latest iteration maintains the performance benefits of the legacy version while also incorporating new advancements.
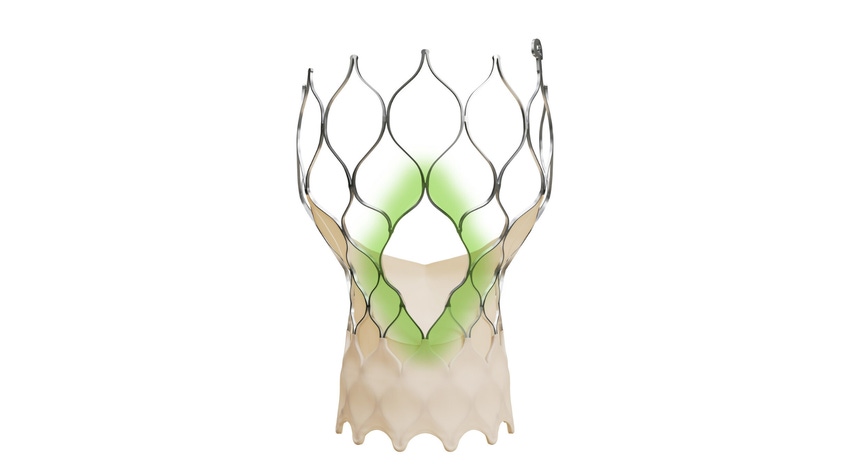
Medtronic today announced that FDA has approved the Evolut FX+ transcatheter aortic valve replacement (TAVR) system for the treatment of symptomatic severe aortic stenosis. The system is the latest iteration of Evolut FX+ which maintains the performance benefits of the legacy version which is designed to facilitate coronary access while also incorporating new advancements.
Severe aortic stenosis happens when the “aortic valve leaflets become stiff and thickened and have difficulty opening and closing, making the heart work harder to pump blood to the rest of the body,” according to the company press release announcing the approval. “Severe aortic stenosis often reduces a patient's quality of life and limits their daily activities. If left untreated, 50% of patients with symptomatic severe aortic stenosis can die from heart failure in as little as two years.”
TAVR is a minimally invasive procedure to replace the aortic valve in patients with severe aortic stenosis, is less invasive than open heart surgery, and typically takes less than an hour to complete. During the procedure, a surgeon will make a small cut in one of three access routes: the groin, the neck, or a space between the ribs. The doctor will then guide a thin, flexible tube with the heart valve into the artery to the diseased valve. Once the valve is placed, it will work immediately.
The Evolut FX valve is made of a blend of nickel and titanium, which allows the frame to shape itself to the anatomy, according to Medtronic. Additionally, the valve frame has gold markers beneath the outer wrap so the doctor can better see it during a procedure.
In the new iteration, Evolut FX+, the system offers larger coronary access windows through a modified diamond-shaped frame design which is four times larger than previous iterations. It also provides increased space for catheter maneuverability for better access to varying patient coronary artery anatomy. The company noted that the new design does not compromise the performance of the valve, its hemodynamics, and radial strength.
The system is indicated for symptomatic severe aortic stenosis patients across all risk categories, including extreme, high, intermediate, and low. Medtronic expects its US early commercial experience to occur in spring 2024, with the full launch anticipated in the summer.
“We are excited to have received FDA approval for the Evolut FX+ TAVR system to treat patients with symptomatic severe aortic stenosis, broadening access of minimally invasive solutions to patients in need of safe and effective treatments,” Nina Goodheart, senior vice president and president, Structural Heart & Aortic at Medtronic, told MD+DI. “This is another major milestone for TAVR and the Evolut platform, and we are proud this new design continues to deliver market leading valve performance for physicians to treat their patients.”
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
