FDA Grants Conditional Approval to Artificial Cervical Disc
Degenerative disc disease is a widespread condition, with up to 85% of the population showing evidence of the disease by age 50. Spinal fusion is commonly used to treat the condition although, in the future, cervical arthroplasty may be used to treat a significant number of such patients.
November 7, 2012
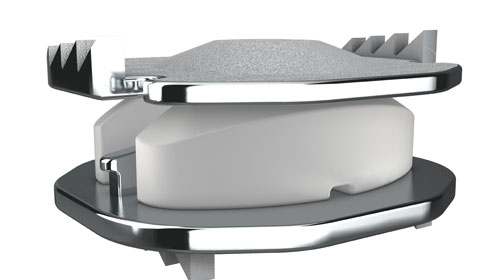
Privately held LDR Medical (Austin, TX) hopes to help drive this trend. The company has recently announced that it has received an approvable letter from FDA for its two-level Mobi-C cervical disc, making it the first cervical disc to receive an approvable letter for two-level use in the United States. The development was the result of a 600-patient concurrent IDE clinical study for one and two-level cervical disc replacement. To learn more about the news, we spoke with Joe Ross, vice president of U.S. marketing at the company.
MD+DI: Could you briefly summarize the clinical trial results?
Ross: LDR is the only company to initiate and completely enroll a concurrent one- and two-level cervical disc replacement trial. There were two arms of the trial running concurrently—one to treat a single spinal disc in the cervical disc in the spine and the other one two treat two disease levels in the cervical spine—to replace one or two discs depending on the patient’s pathology and how they presented. We completed enrollment and submitted both of those data sets to the FDA and they were under review by different teams. Due to the timing of the reviews, the FDA approval for the two-level cohort has preceded the letter for the single level cohort.
I can’t make any claims based on the data because we are not yet approved. I can really only say that our investigators are extremely pleased with their review of the data to date. We are obviously very enthusiastic about what we are able to see. We are really looking forward to the publication of the data in the peer reviewed literature and ultimately the final review and approval of claims by the FDA.
MD+DI: It may be a bit early to tell conclusively but how does cervical disc replacement compare in terms of clinical efficacy perspective to fusion?
Ross: The control treatment for our trial and all of the cervical disc trials to date is to compare cervical disc replacement to anterior cervical discectomy and fusion or fusion procedure. Most of the conclusions for the other trials to date have been that artificial disc replacement at two years for single level is at least non inferior to cervical fusion. The community has been very interested in looking at longer term results. Interestingly, much of the literature hints at favorable trends for artificial disc replacement but those are not part of available claims. I will point to the recent approval of the Secure-C by Globus, which actually has a superiority claim over fusion at two years. The clinical results overall appear very promising. LDR and the other companies [involved in this space] are interested in continuing to gather long-term results.
The investigators in our trial are very happy with the results that they have seen. We are, however, not at the point yet where we can make claims based on the data because we don’t yet have full approval.
MD+DI: How big is the potential market for artificial cervical discs?
Ross: The estimate I have heard from analysts and leading physicians point to roughly 250,000–275,000 anterior surgical procedures performed in the United States annually. This is a very broad range but in terms of the percentage of those patients who may be appropriate for cervical disc replacement, it could be anywhere from 25 to 40 percent of those patients long-term. It’s nowhere close to that now of course; it is still very early.
MD+DI: How would you describe the interest in orthopedic surgeons in the device?
Ross: Again, we are not on the market in the U.S. We have been selling the Mobi-C surgical disc outside of the U.S. since 2004. LDR is present in close to 30 countries across the world. Outside of the United States, Mobi-C is our number-one-selling product. We receive a very enthusiastic response to cervical disc replacement outside of the U.S. Domestically, I think that there is significant enthusiasm for the potential of the technology. However, it has been tempered to date by the availability of both broad coverage by the payor community and also limited availability variety of newer technologies over what has to date been available.
MD+DI: For those who may not be familiar with the company, could you provide a brief summary?
Ross: LDR, the name of the company is the first letter of the three founder’s last names: Christophe Lavigne, Hervé Dinville, and Patrick Richard. The three men founded the company in Troyes, France in 2000 and began developing products and initially went to market in Europe in 2002.
They had a focus on some pretty unique technologies including the Mobi platform of artificial disc replacements. They began with Mobi-C in 2004. The U.S. office was opened in Austin, TX in 2005 primarily to support the IDE trials of the Mobi-C. More recently, we have been able to grow the company and increase our presence in the U.S. market with a strong focus on our VerteBridge plating technology platform of interbody cages including ROIC and ROIA standalone anterior cervical and lumbar cages and more recently the introduction of the Avenue L lateral lumbar cage.
Brian Buntz is the editor-at-large at UBM Canon's medical group. Follow him on Twitter at @brian_buntz.
You May Also Like


