Achieving Medical Device Quality Management System Excellence
By addressing regulations associated with an FDA abbreviated inspection, organizations can enhance product performance, comply with regulatory standards, and ensure customer satisfaction.
July 20, 2023

Michael Kuehne, ComplyFDA.com
A robust Quality Management System (QMS) for medical device manufacturers is a critical foundational element of high-quality manufacturing and distribution. The QMS, as defined by regulatory standards such as ISO 13485 and 21 CFR Part 820, is a cohesive system that oversees all elements of a medical device's lifecycle, from concept and design to manufacturing and post-market surveillance.
The effectiveness of the QMS directly affects medical device performance and safety, and, subsequently, customer satisfaction. This connection underscores the necessity of meticulously managing all aspects of it.
Reliable, data driven quality management system best practices can greatly improve the overall QMS performance, cost effectiveness, and compliance with applicable medical device regulations. Such best practices are utilized by high performing organizations of all sizes and years of operation to evaluate QMS gaps and implement sound improvements or, in the case of small organizations, provide a strong backbone from which to build a QMS appropriately sized for the particular requirements relative to the size of the organization and device class of products produced.
A major consideration for this is compliance to both 21 CFR 820 and ISO 13485 regulations established for medical device manufacturers. Regulatory compliance audit performance is a key success factor which can be time consuming and costly for organizations that perform poorly during regulatory audits.
Figure 1:
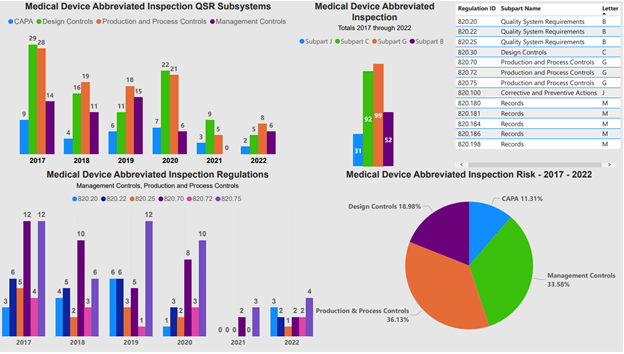
Figure 1 shows both the medical device QMS subparts and the individual 21 CFR 820 regulations associated with an FDA abbreviated inspection.
The critical areas identified in Figure 1 significantly impact product performance and customer satisfaction — design control, risk management, document control, supplier quality management, production and process controls, corrective and preventive actions (CAPA), training, equipment maintenance and calibration, change management, complaint handling, internal audits, and management review. While not the only areas reviewed by regulators and customers during customer audits, these are the most common areas for review.
These areas form the foundation of a solid QMS typically demonstrating higher performance levels when the systems are based on reliable, data driven input. A failure in any of these can lead to poor product performance and dissatisfaction among customers, while mastery of these areas leads to a seamless manufacturing operation and superior product quality.
The most critical areas of a medical device QMS include:
Design Control: The design process is where product specifications and requirements are defined. Medical devices should be designed with the end-user in mind and follow a formal development process. This includes defining user needs, translating these into design inputs, developing the design, and validating it with design verification and validation processes. An insufficient or flawed design control process can lead to a product that fails to meet user needs or regulatory requirements, thus compromising product performance and customer satisfaction. A major obstacle to success is the lack of a clear understanding of user needs or requirements. This could lead to a design that fails to meet intended use or regulatory requirements.
Risk Management: Risk management in a QMS involves identifying, assessing, and controlling product and process risks throughout the entire product lifecycle. This includes both risks to the patient and risks to the quality of the product. The ISO 14971 standard provides guidance for risk management for medical devices. An ineffective risk management system could overlook potential hazards, which might result in device failures, harm to patients, or significant financial losses. Identifying and assessing all potential risks can be challenging, as it requires a multidisciplinary approach and a deep understanding of the product and its use. One of the biggest obstacles in risk management is predicting all potential risks that may arise throughout the product lifecycle. This involves understanding the clinical use of the product, patient population, the environment in which the device will be used, and the potential hazards. As medical technology continues to evolve, new types of risks can emerge. An organization needs to employ a systematic approach and have a team with a diverse set of skills — clinical, engineering, regulatory — to conduct a comprehensive risk assessment.
Document Control: This system ensures that all documents required for product design, production, and post-market surveillance are correctly created, reviewed by all key stakeholders, approved, and maintained. This includes Standard Operating Procedures (SOPs), work instructions, forms, records, policies, and specifications. Inadequate document control can lead to miscommunication, misunderstanding, and mistakes during production and other processes. The sheer volume of documentation can be overwhelming, and maintaining their relevance, accuracy, and accessibility over time is a key success factor.
Supplier Quality Management: Suppliers can significantly impact the quality of the final product. Therefore, a robust supplier management system is needed. This includes qualifying suppliers, monitoring their performance, conducting audits, and addressing any non-compliances. Poor supplier quality management can lead to substandard components or raw materials, which can affect the final product's quality and performance. Diverse suppliers with different standards and practices can complicate the task of ensuring consistent quality across all inputs increasing the importance of a robust supplier management system based on established best practices. With a global supply chain, managing supplier quality is increasingly complex. The diversity among suppliers in terms of their quality systems, standards, practices, and cultural factors can make it challenging to ensure a consistent level of quality. Changes in the supplier's process or management, which are beyond the control of the organization, can impact the quality of supplied components or materials. Organizations need to have a robust supplier management strategy that includes qualifying and classifying suppliers based on the risk, regular supplier audits, performance monitoring, and managing non-conformances. Developing a strong relationship and open communication with suppliers can also help in managing supplier quality effectively.
Production and Process Controls: These ensure the production process consistently produces quality products. They involve defining process parameters, monitoring the processes, controlling product non-conformities, and ensuring equipment and environmental conditions are controlled. If not properly managed, variations in the production process could lead to defects, recalls, or poor product performance. Variability in production processes is a significant hurdle. Controlling all variables to ensure a consistent output is a key performance aspect of high performing organizations. The biggest challenge in this area is managing the variability that can arise in production processes. Variables can range from material inconsistencies, equipment wear and tear, and environmental changes, to human error. Managing this requires a deep understanding of the production process and a well-developed process control strategy. This often involves statistically based methodologies such as Six Sigma or Statistical Process Control (SPC) to monitor and control the process. It's a continual process of data collection, analysis, and making adjustments to ensure the process stays within defined limits.
Corrective and Preventive Actions (CAPA): The CAPA process is used to investigate and rectify the root causes of detected problems. This includes nonconformities in the product, process, or QMS. An ineffective CAPA process might fail to identify or correctly address the root causes, leading to recurrent issues and poor product quality. Identifying the true root cause of a problem can be challenging, and without this, actions taken might not fully address the issue. Root cause analysis is a thorough investigation that seeks to understand the fundamental, underlying issue that caused a non-conformity, rather than simply addressing the apparent symptoms. Without a correct root cause, any actions taken might not fully resolve the problem, leading to recurrence. Determining the root cause can be complicated by a multitude of factors including complex processes, interrelated factors, or a lack of clear evidence pointing to the cause. Once the root cause is determined, designing effective corrective or preventive actions can be difficult. These actions should be proportionate and targeted, addressing the root cause without adversely affecting other areas of the system. They should also be feasible in terms of cost, time, and resources. After implementing a corrective or preventive action, it is essential to monitor the situation to confirm the action has resolved the issue and hasn't resulted in unintended negative consequences. This requires a well-planned monitoring strategy based on sound metrics and an adequate timeline, as the impact of CAPA might not be immediately evident.
Training: Employee training is essential to ensure everyone understands their role in the QMS, how to perform their tasks correctly, and the importance of quality. Poor training can lead to mistakes during production, non-compliance with procedures, and ultimately, quality issues. Providing relevant, up-to-date training to all employees in a timely manner, especially in an ever-changing environment, mitigates the risk of human performance errors.
Equipment Maintenance and Calibration: Regular maintenance and calibration of equipment is essential to ensure it functions as intended. Poorly maintained or uncalibrated equipment could produce substandard products, negatively affecting product performance. Regularly scheduling and carrying out maintenance and calibration for a large number of devices, each with its own requirements, can be a logistical challenge. Use of automated systems for monitoring and scheduling maintenance and calibration are core elements of high performing organizations.
Change Management: Change management involves planning, implementing, and monitoring changes in the product, processes, or QMS to ensure they don't negatively impact product quality. Poor change management can introduce new risks or problems, affecting product performance and customer satisfaction. Balancing the need for change (for improvement or adaptation) with the risk that change might disrupt a currently well-functioning system is a characteristic of a robust change management process. The primary challenge in change management lies in balancing the need for change and maintaining the integrity of existing systems. Change, while often necessary for growth, improvement, and adaptation to new regulations or technologies, can disrupt the current processes if not managed properly. A poorly managed change can lead to unintended consequences such as a decline in product quality, increased risks, or non-compliance with regulatory requirements. It's crucial to have a structured change management process, which includes a thorough impact assessment, risk analysis, communication, and training plan.
Complaint Handling: Effective complaint handling is essential for addressing customer dissatisfaction promptly and preventing similar future issues. It provides a means to identify product and process issues post-market. Failure to manage complaints can harm the brand's reputation, lead to regulatory action, and reduce customer satisfaction. Rapid and effective resolution of customer complaints, especially when they involve complex technical or medical issues is critical to customer satisfaction.
Internal Audits: Regular internal audits should be conducted to check the compliance of the QMS with the defined procedures and regulatory requirements. Audits can identify areas for improvement, and the lack of such a system could mean undetected non-compliances or potential issues, leading to quality problems down the line.
Management Review: Management reviews should be conducted at planned intervals to ensure that the QMS is suitable, adequate, and effective. This includes reviewing quality objectives, audit results, CAPAs, and customer feedback. If not conducted correctly, management might not be aware of existing issues or necessary resources, which could impact the effectiveness of the QMS and, subsequently, the quality of the product. Ensuring that top management is fully engaged, understands the importance of the QMS, and is willing to provide necessary resources presents unique challenges as organizations balance quality and compliance with resource allocation and cost effectiveness. A streamlined, compliant QMS operating in accord with validated best practices is invaluable to organizations wishing to strike the appropriate balance between quality, compliance, resources, cost, and profitably providing life enhancing products.
QMS in medical device manufacturing is a comprehensive framework that ensures consistent product quality and safety, ultimately driving customer satisfaction. The critical components identified in this report, from design control and risk management to internal audits and management review, play a vital role in shaping QMS and influencing product performance.
Failures in any of these areas can lead to compromised product quality, risk to patient safety, regulatory non-compliance, and reduced customer satisfaction. However, by adequately addressing these critical areas, organizations can significantly enhance their product performance, comply with regulatory standards, and ensure their customers' satisfaction.
Proper management, constant vigilance, and a culture of quality are essential to sustain a robust QMS. This approach not only mitigates risks but also promotes continual improvement — a journey towards excellence where the destination is as important as the journey itself. By paying close attention to these facets of quality management, medical device manufacturers can continue to provide life-enhancing, and often life-saving, products to the patients who need them.
For more information or to learn more about support for medical device Quality Management Systems contact:
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
