Type II Diabetes and Obesity are the dual targets of this non-surgical implantable medical device
People suffering from Type II diabetes and related obesity may have a new medical device that addresses both the chronic conditions at the same time.
People suffering from Type II diabetes and related obesity may have a new medical device that addresses both the chronic conditions at the same time.
Lexington, Massachusetts based GI Dynamics has developed an endoscopic, temporarily implantable device that is placed inside a person's gut that can faciliate better blood sugar control and reduce weight, said the company's CEO Stuart Randle in a recent interview.
|
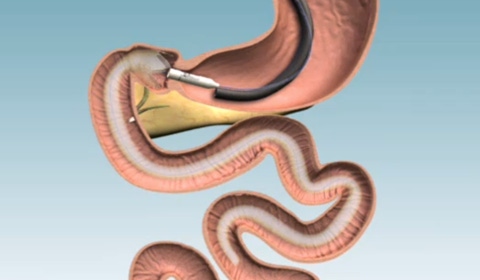
The EndoBarrier is a thin liner placed endoscopically |
The implantable devic, known as EndoBarrier, is a thin liner that is inserted through the mouth using a tube and secured just below the stomach and at the beginning of the intestine. As food passes through the EndoBarrier it doesn't come into contact with the rest of the stomach including digestive enzymes. As a result, the EndoBarrier lengthens the time needed to digest food in effect keeping the diabetic/obese person feel full for a longer period. The device is left in the body for 12 months after which it is removed. The temporary nature of the EndoBarrier is what Randle believes will appeal to patients because even though bariatric surgery works, patients are reluctant to have such a major surgical procedure done that alters their body forever.
"The U.S. has the highest penetration rate of bariatric surgery in the world and it still is less than one percent," Randle says. "Patients are concerned about the risk of surgery, or they don't want to have surgery to have their anatomy permanently re-plumbed if you will. They don't want to have a permanent implant."
Add to that the fact that with EndoBarrier most patients can go home the same day, the risks and complication rates are much lower, not to mention much lower procedure costs, it makes the device a more attractive option, he declares. It is aimed at people who have diabetes and have a Body Mass Index ranging from 30 to 50.
EndoBarrier is currently being tested in the U.S. in a blinded clinical trial but it is approved for sale in many regions including in Europe and Australia. GI Dynamics, which is listed in the Australian Securities Exchange plans to expand in Asia, Europe and South America this year and has struck distribution agreement with a company in Brazil while the clinical trial ramps up in the U.S. The company had around $41.5 million in cash at the close of last year, Randle says.
What makes GI Dynamics unique is that early on, the company transitioned to having a primary focus on glycemic control to manage diabetes and secondarily on treating obesity, Randle says.
"There have been over the last decade 50 to 100 companies in the obesity space - device companies," Randle says. "And we really transitioned about five years ago to a primary focus on diabetes and a secondary focus on weight loss."
While there are other companies that are treating weight loss in non-surgical ways, there is only one that Randle is aware of that like EndoBarrier employs a liner in the intestine. That company is based in Chicago, but the company's website doesn't show any information after October 2009. A search of the ClinicalTrials.gov shows that Valentx appears to have completed a feasibility trial in Mexico in 2010.
GI Dynamics may be bringing the therapy to locations overseas, but U.S. patients have to wait a while. The company expects to file a premarket approval application with the FDA in late 2015.
- By Arundhati Parmar, Senior Editor, MD+DI
Related Content
Digital Health Startup Focuses on Diabetes Prevention
Global Gastrointestinal Devices Market to Grow to $33 billion in 2023
About the Author(s)
You May Also Like



