Smart Artificial Urinary Sphincter Advances Bid for FDA Approval
UroMems meets the six-month primary endpoint for the “first-ever” female implanted with its automated artificial urinary sphincter.
At a Glance
- UroMems achieves a milestone as the first female patient implanted with the UroActive System meets primary endpoints.
- UroMems addresses pain points associated with current technology for stress urinary incontinence.
- The company is in contact with FDA for the next steps and to achieve potential approval as quickly as possible.
UroMems reported it met the six-month primary endpoint for the “first-ever” female implanted with the UroActive System.
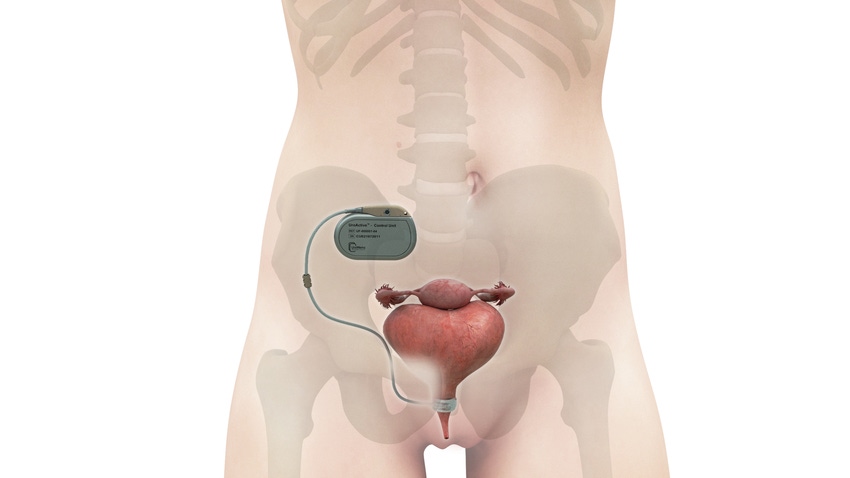
The UroActive System, the first smart automated artificial urinary sphincter to treat stress urinary incontinence, is designed to overcome pain points associated with currently available technology used to treat the condition, according to UroMems CEO and Co-founder Hamid Lamraoui.
Studies to Date
The news of the first female patient comes after the company, based in Grenoble, France, and Minneapolis, Minnesota, announced successful results from its male cohort clinical feasibility study.
These are among the six male and six female patients in the company’s two pilot studies, which are prospective, open-label, non-randomized, multi-center, single-arm trials. Primary outcome measures are the rate of explants and revisions at six months after device activation and the rate of device activation successes. The main secondary outcome measure is the number of subjects with a 50% reduction or greater in 24-hour pad weight test at 90, 185, and 365 days after activation.
In December, UroMems announced the complete treatment cohort of six men in the first-of-its-kind clinical feasibility study had reached the six-month primary endpoints.
“All six men are now implanted for at least seven months and up to 15 months, with their devices operating as expected and no need for revision nor explant. In addition, extremely positive follow-up was received on secondary outcomes measures, including leak rate values and patient quality of life questionnaires,” according to a company press release.
The first female patient met the study’s primary endpoints and according to UroMems, is experiencing restored social continence.

UroMems CEO and Co-founder Hamid Lamraoui
Continued successful results will lead to UroMems' pivotal clinical trial in Europe and the U.S. and a potential FDA approval through the agency’s Safer Technologies Program (STeP) designation, which aims to shorten the time to develop and obtain marketing authorization for eligible devices.
Fixing Pain Points
Known shortcomings associated with the approved artificial urinary sphincter device and industry standard treatment for severe stress urinary incontinence in men, the AMS800 (Boston Scientific), have created the need for innovation. [source: Artificial urinary sphincter: current status and future directions - PMC (nih.gov)]
Lamraoui told MD+DI that he learned of the challenges associated with available artificial urinary sphincter more than a decade ago while talking with Professor Pierre Mozer, MD, PhD, a Paris, France urologist and UroMems CMO and co-founder.
“… he was seeing so many patients who were suffering from [severe] stress urinary incontinence without having the right solution for them. This is for many reasons,” Lamraoui said.
The concerns prompted the International Continence Society to create an expert panel in 2015 to reach a consensus on diverse issues related to the AMS800 device. Their recommendations were published in 2016.
Among the primary challenges with AMS800, according to Lamraoui, is that to activate the implanted AMS800, patients have to manually pump it several times. This can become a nightmare for especially females, who are likely to pee on their hands in the process, he said.
UroMems eliminated the need to manually pump the device by replacing the manual pump in the body with a patient-activated remote control, allowing patients with the UroMems to void and control the level of device pressure while using it.
Another challenge with the existing technology is that it cannot be personalized to the patient. Its occlusive cuff is placed around the urethra or bladder neck and, when inflated puts pressure on the urethra to stop voiding or releases it to void. The issue with the AMS800 is not with the occlusive cuff, itself, but rather in how it generates the right pressure for an individual patient, he said. AMS800 is a passive hydraulic device that transmits urethral occlusion pressure by its pressure-regulating balloon, or PRB. PRB pressure is a fixed (usually 61-70 cm H2O as a consensus-validated value) and cannot be adjusted after implantation, according UroMems.
With the AMS800, surgeons select one of three options during surgery. If they select the wrong option for the patient, then either the patient will continue to have leaks (low pressure) or the pressure will be too high, leading to atrophy or erosion of the urethra, according to Lamraoui.
“… our device is a smart device, meaning, first, we can fully personalize it. You can program the pressure for every patient, and this is done after surgery with wireless communication by a tablet that is easily used by the physician to titrate the device,” Lamraoui said.
A MyoElectroMechanical System (MEMS) powers UroActive technology. Placed around the urethral duct, MEMS automatically controls the device based on the patient’s activity, without the need for manual adjustments. Theoretically, this should lead to increased urethral pressure when a person is engaged in a physically demanding activity and less compression during more sedentary times, potentially reducing tissue damage, such as erosion.
Lamraoui also noted the lack of data from the AMS800.
“This is something you implant in the patient, and you don’t have any communication or anything coming from the device. So, in a way the surgeon is blind with those solutions,” he said. “We have a sensor inside the device and are able to report all the events coming from the device. We are able to retrieve the information.”
These pain point solutions, UroMems hopes, will lead to better efficacy and patient and physician experiences.
Lamraoui said they are in contact with FDA for the next steps and to achieve potential FDA approval as quickly as possible.
“Today with the resources we have there is no reason to slow down the project and actually are accelerating things because we have such good results that there is no reason to wait,” he said.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
