August 22, 2023
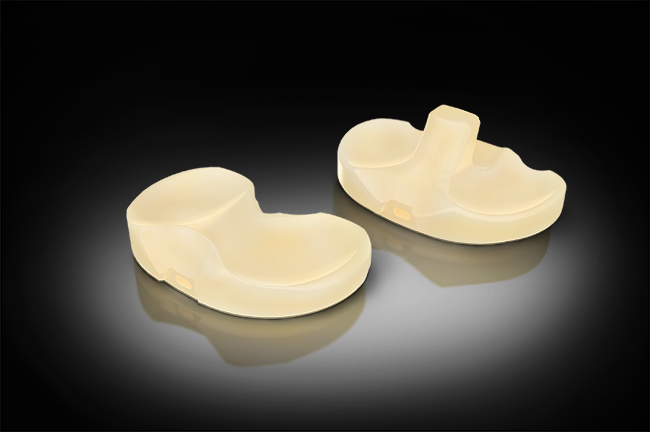
Orthopedic implant and instrument company Exactech announced on Aug. 21 that it has received 510(k) clearance from FDA for its new Activit-E polyethylene (PE) for the Truliant knee-replacement system. The new PE with vitamin E antioxidant does not require gamma sterilization, unlike previous formulations of the material.
“After years of research and development in polyethylene, Activit-E represents a breakthrough achievement for Exactech,” said Adam Hayden, chief marketing officer and senior vice president of the Large Joints Business Unit at Exactech. “It is the next generation of highly cross-linked polyethylene with vitamin E antioxidant that is intended to further our primary goal of providing immense benefits to our patients.”
|
The chemically cross-linked polyethylene brings strength, toughness, oxidative resistance, and long-term performance to the Truliant knee-replacement system. |
The chemically cross-linked PE reportedly brings an optimized balance of material strength and toughness without requiring the use of gamma sterilization. The material is designed to maintain active oxidative resistance and long-term performance, including strength and stabilization, according to Exactech.
The PE was developed by Orhun Muratoglu, PhD, director of the Harris Orthopaedic Laboratory at Massachusetts General Hospital in Boston. Muratoglu invented the first cross-linked PE, and the first of multiple generations of vitamin E, antioxidant PE for leading orthopedic companies, said the news release.
“We replaced gamma radiation cross linking with peroxide cross linking and stabilized the poly with vitamin E to provide strength, flexibility, and toughness where it is needed most — total knee arthroplasty,” said Muratoglu. “This technology also addressed the looming shortage of gamma radiation for cross-linking, ensuring that patients will continue to benefit from the clinically proven advantages of highly cross-linked polyethylene in total joints,” said Muratoglu.
As reported in sister media outlet MD+DI, in recent years there has been tightness in the supply of cobalt-60, the radioactive isotope that generates the gamma radiation used to sterilize healthcare products. Core to the process, cobalt-60 is produced in a handful of nuclear reactors and specialized suppliers. The shortage is a result of increasing demand and the decision of one state-run corporation to reduce output, reported MD+DI.
Activit-E launched in the beginning of Q3 2023 for select US customers, with global expansion set to start in 2024.
Based in Gainesville, FL, Exactech develops and markets orthopedic implant devices, related surgical instruments, and the Active Intelligence platform of smart technologies to hospitals and physicians.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
