Test May Predict HIV Drug Resistance
March 1, 2007
R&D DIGEST
|
From left, Haifeng Chen, Fangping Cai, and Feng Gao have developed a sensitive test that can detect genetic mutations in HIV that cause drug resistance. |
Researchers are trying to tackle predicting a patient's resistance to HIV drugs with a very sensitive assay. Genetic mutations of HIV can occur rapidly, making the virus more resistant to drug treatment. Researchers at Duke University Medical Center (Durham, NC) have developed a test that can detect these mutations, which could also help doctors predict a patient's resistance to HIV drug treatment. The technology may also have promise for detection in other treatment areas.
What sets the assay apart is its sensitivity, which means the test can detect the mutation down to 0.1–0.01%. The test is about 1000 times more sensitive than a conventional genotyping assay, says Feng Gao, MD, associate professor of medicine at Duke's medical center. It can detect a single mutated virus out of 10,000 nonmutated viruses in a sample. The test can also identify when a virus molecule has more than one mutation, something that no test on the market can do, says Gao.
The assay looks for genetic mutations at specific areas that are linked to drug resistance. During test evaluation, researchers examined blood samples from three groups of HIV patients. They looked at those who had never received antiretroviral drugs, patients who had been given the drugs in the past, and patients who received the treatment without complete success.
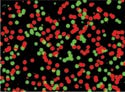
The researchers isolated the genetic material in the blood samples. Then they used fluorescent tags that stick to HIV genes to identify the mutation. The tags sticking to the mutated genes known to cause drug resistance were green, and the nonmutated genes in the same locations were tagged red. A computer program counted how many green and red tags were in each sample.
|
Genes tagged green demonstrate resistance, while those that are tagged red do not. |
The researchers are interested in licensing the technology, but they say such licensing needs to happen fast for commercial viability. Automating the test would cut both labor and cost. “We're in an academic setting, so everything that's been done in that lab has been manual,” says Gao. The test currently takes about two weeks to analyze 30 primary resistant mutations. If fewer mutations are required for testing, the time can be much shorter, he says.
The test also might be able to help those with other diseases. Broader applications could include detecting mutations related to cancer, which could help in prognosis or diagnosis, and finding mutations that indicate drug resistance in hepatitis B, hepatitis C, and tuberculosis.
The researchers' findings appear in the February edition of Nature Methods. Fangping Cai, Haifeng Chen, Charles Hicks, John Bartlett, and Jun Zhu also worked on the test. Duke has filed a provisional patent on the technology, and the researchers are looking into either licensing it to a company or starting their own firm to continue developing the test. The National Institutes of Health and the Duke Center for AIDS Research supported their work.
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)

