Exploding Microcapsule Material Developed for Drug Delivery
February 16, 2012
|
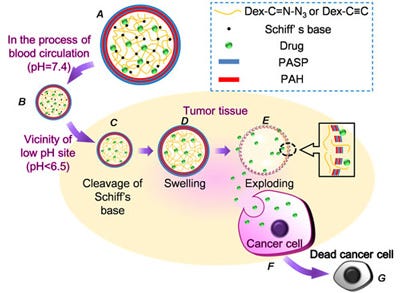
Structure of a microcapsule and tumor-triggered explosion of the microcapsule. A) Structure of microcapsules, B) microcapsules host drugs at pH 7.4, C) Schiff's base cleaves at low pH site, D) increased pressure causes the microcapsule to swell, E) exploding of the microcapsule, F) uptake of drugs released from exploding microcapsules by cancerous cell, and G) apoptosis of tumor cells. Image: Dr. Xian-Zheng Zhang, Wuhan University/Nanowerk.com. |
For a nano- or microscale drug-carrier system to be effective, it needs to identify and reach its target. It also needs to release its payload at the target at the right time or over a certain period of time. This controlled release, however, has proven to be a challenging issue, reports Nanowerk.com in its recent Nanowerk Spotlight piece on exploding microcapsules used to treat cancer.
Typically, some of the drug is released in the bloodstream before it reaches the target cells. "It is of great importance to design intelligent drug carriers that can specifically respond to physiopathological signals and allow explosive release of the loaded drugs while entrapping the drugs efficiently during the process of blood circulation," Xian-Zheng Zhang, director of the Key Laboratory of Biomedical Polymers of Ministry of Education and a professor in the department of chemistry at Wuhan University in China, told Nanowerk. To avoid the side effects of prematurely released toxic cancer drugs on healthy tissues, Zhang and his team designed and fabricated an "active defense" system and material that could effectively keep the drug entrapped in its carrier in the blood and normal tissues but allow an explosive drug release under the right physiopathological stimuli once the drug carrier reaches the cancerous tissues.
"As far as we know, developing satisfactory drug-delivery systems which could explosively release the drug in response to environmental stimuli still remains a great challenge," said Zhang in the Nanowerk story.
Zhang's researchers demonstrate an "active defense" system that allows the explosive drug release once the drug carrier is activated by the environmental stimuli present at the cancerous tissues. The team reported its findings in the February 2, 2012 online edition of Advanced Functional Materials.
To make their microcapsules, the scientists first prepared biodegradable dextran microgels crosslinked by a Schiff base. Then they coated the surface of the microgels with a layer-by-layer membrane. Once in blood circulation, the system is activated by the physiopathological stimuli - an acidic environment - at the cancer site: the Schiff base hydrolyzes in acidic environments. This hydrolyzation causes the crosslinkages containing C=N bonds to be cleaved, leading to increased swelling pressure of the dextran core. When the swelling pressure is higher than the membrane can resist, the surrounding layer-by-layer membrane will rupture followed by an explosive release of the entrapped drug, which is then taken up by the cancer cells.
In a previous study, Zhang's team prepared microcapsules that could release a drug rapidly under physiological conditions upon addition of dithiothreitol (DTT). Upon the addition of DTT, the crosslinkages containing disulfide bonds are cleaved, leading to increased swelling pressure of the dextran core. When the swelling pressure is higher than the membrane can resist, the surrounding layer-by-layer membrane will rupture, followed by an exploding release of the entrapped drug. The need for an external trigger - which needs to be injected at the tumor site - is the limitation of this system.
Zhang cautions that his newest system still has some drawbacks with regard to practical use, such as the relatively large size of the microcapsules and a lack of passive targeting properties. But he believes the drawbacks can be overcome by changing the size of microcapsules, incorporating PEG to prolong the blood circulation period, and incorporating functional peptide sequences such as RGD to endow with targeting properties.
About the Author(s)
You May Also Like
.png?width=300&auto=webp&quality=80&disable=upscale)

