Designing Next Gen Surgical Energy Systems
Dan Friedrichs shares perils, pitfalls, and possibilities.
There are more than 300 in-market devices that deliver radiofrequency, microwave, electric field, or ultrasonic energy for use in medicine or surgery. Clinical uses include ablation, surgical cutting and cautery, neuromodulation or denervation, tissue remodeling, vascular closure, and more. While the clinical applications are widely variable, one thing all these devices have in common is that their designs will eventually become dated, their parts obsolescence issues will accelerate, impacts of changing regulatory standards will become more severe, and user needs and preferences will evolve. These situations call for a product refresh.
Evolving an existing device into its next generation is an important investment decision that enables OEMs to lead their markets with device enhancements in functionality, scalability, and usability. Because of the impacts on the company, next-gen programs must align with business dynamics, market/user feedback, and ROI. Here are five key considerations to keep in mind at this critical juncture:
Predicate equivalence
Are you planning to use your existing system as a predicate? If so, are you sure the system performs as you think it does? Many next generation development efforts get derailed when it is discovered than a predicate system doesn’t meet its specs or original design requirements or projects get extended when the bugs of the first-generation device need to be carefully designed into the next generation system for equivalence. Do you have the capability and knowledge to characterize your existing product, and do you have strong confidence in its performance? How does performance vary unit-to-unit or over operating conditions?
The first step in a next-generation product design should be careful assessment of the existing product, using specialized measurement equipment and procedures, such as the equipment being used to characterize an ablation system’s pulsed RF performance.
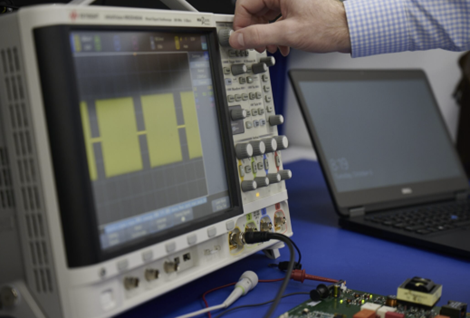
An extensive analysis of your existing product may seem like an odd first step in the design of your new product but, in most situations, it’s an effort sure to pay for itself manyfold.
Futureproofing and extensibility
Given the costs of developing a new medical device, it’s logical to try to anticipate future needs and design features to support them. A balancing act exists, however, how do you avoid burdening a design with future proofing that ends up never being used while still seeing the future clearly enough to find tangible ways to add extensibility? This is where an experienced design partner can make a tremendous difference. Consider the value added by these two design decision examples:
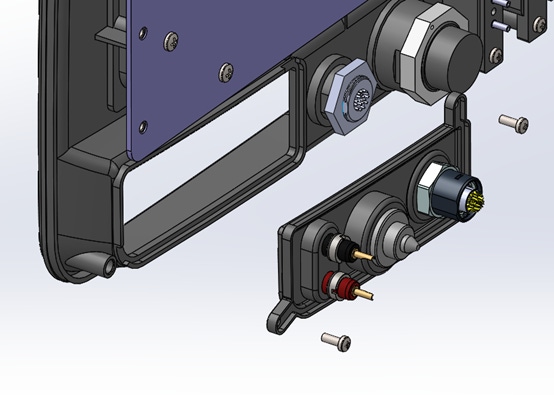
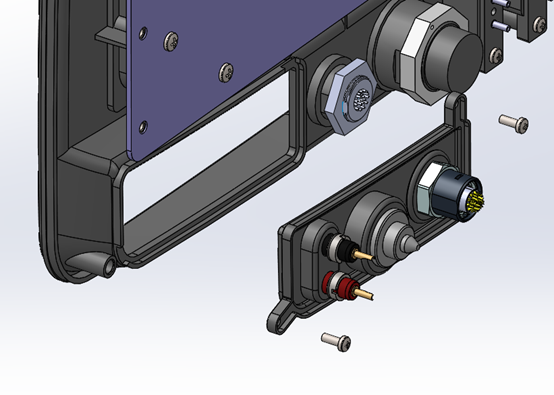
Modular connector panels: Surgical energy devices usually interface with catheters or instruments that have their own product lifecycles to consider. One prudent approach to ensuring future proofing is to define the connection interface in a manner that facilitates future changes with minimal disruption to the rest of the system. As an example, the system below placed a set of connectors that was planned to be deprecated in the future onto a removable panel. This allowed for a service depot-level change that had no impact on the expensive screen, bezel, or remainder of the system.
Upgrade planning: Foresight applies to electrical design as well. On the below circuit board, a low-cost System-on-Module was designed in but was near the limits of its capabilities at product launch. Designers included electrical and mechanical provisions to transition to a larger, higher-performance module, should future software upgrades require additional capability.

This design foresight is an example of no-cost, no-impact early-stage work that could save major redesign costs in the future. Are these types of details being planned for in your new product design?
Cost targets and volume production
Designs that are quick-to-market (common in first-generation products) are rarely low-cost and scalable from a manufacturing perspective. A second-generation product is a rare opportunity to address this through Design For Manufacturability (DFM) and careful tradeoffs of engineering effort versus unit costs.
While DFM is valuable for yield and manufacturing throughput, an often-overlooked value is what it can do for unit cost reduction. A key principle of DFM is “less is more” — fewer parts mean fewer potential failure points, fewer tolerances to stack, and fewer vendors to manage. Is your team considering how they can best simplify the design, before amplifying the impact?
Component availability and supply chain
Supply chain problems grow from seeds planted in the design phase. Ask your design team questions like:
Are you considering risks associated with using special-purpose parts which may have few (if any) drop-in replacements?
Are you intentionally designing for manufacturability and flexibility?
What trade-offs are being made, not just between the usual suspects (size, cost, performance), but also considering longevity, availability, and sourcing?
Design teams seek to optimize, but optimum has different meanings. A key component of an RF ablation generator, an isolation transformer, could be optimally designed in various ways. For a piece of medical capital equipment, which of these two options would you choose?
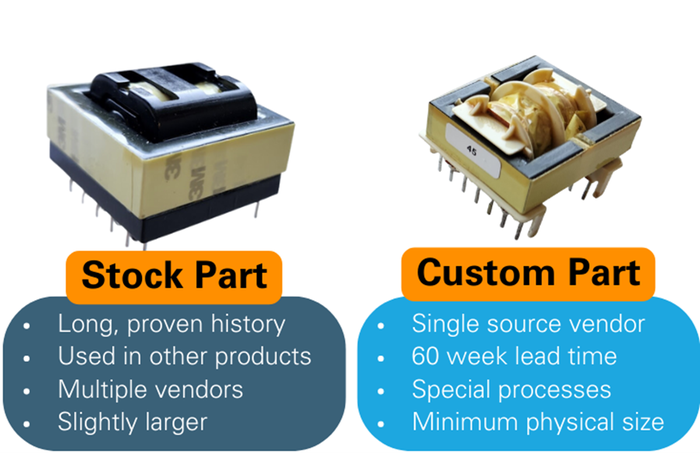
With component availability gating manufacturing across industries, it’s ever more critical to pre-empt these problems, and that means starting to think about them at the early design stage.
Right-sizing your team
Finally, the need for a next-generation device is often an indication that a company, therapy, or business unit is experiencing rapid growth. Is this a chance to re-think partnerships, contracts, and in-house capabilities? While design and manufacturing might still be best outsourced to a competent partner, growth may be a trigger to consider adding other in-house capabilities. Consider how the needs and risk tolerance of a start-up company may differ from a growth-stage company, and what level of in-house capabilities apply to each:
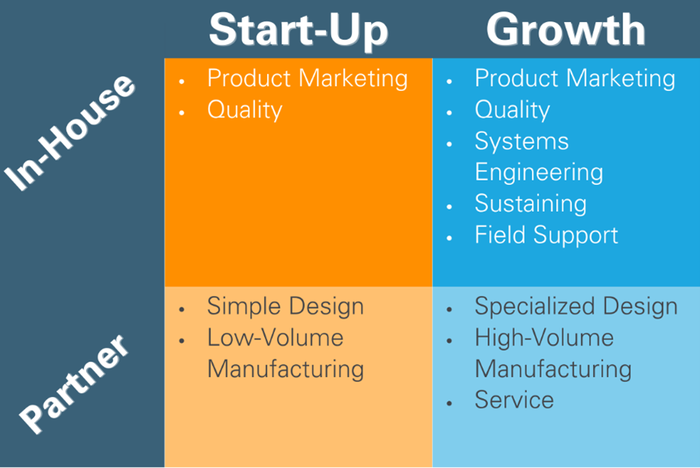
Next-generation products present a great opportunity to assess if existing partners are meeting your needs and if your in-house competencies are aligned.
Conclusions
Next-generation surgical energy systems are often more challenging to bring to market than their predecessors, for reasons that may not be obvious to the uninitiated, but they don’t have to be. With an experienced design team lending fresh knowledge and foresight while managing risks, you can set your company up for continued success.
Daniel Friedrichs, PhD, leads development engineering efforts at Minnetronix Medical, a design, development, and manufacturing partner to leading device companies around the world. He is named on more than 35 patents and has worked with an extensive network of clients seeking to commercialize a wide array of technologies.
Unless specified, all images were provided by Daniel Friedrichs.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
