Behind the Design of the Award-Winning Skytrofa Auto-Injector
Phillips-Medisize was recently recognized with a third industry accolade for the Skytrofa drug-delivery device.
August 30, 2023
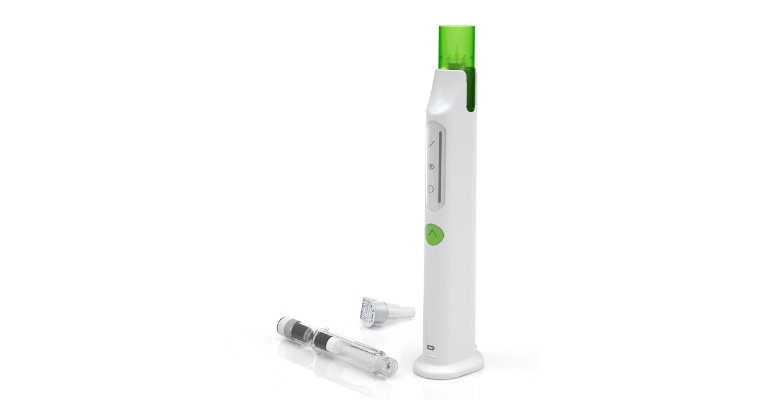
Phillips-Medisize, in collaboration with its customer, Ascendis Pharma, was recently recognized with a third industry award for the Skytrofa Auto-Injector.
The Red Dot Award recognizes the drug delivery device's exceptional design and positive impact. The device is designed to enable convenient and efficient preparation and delivery of Skytrofa, a growth hormone treatment for children. The Red Dot jury praised the device for its ease of use and optics, as well as convenient and safe process control with reduced pharmaceutical waste.
Traditionally, children requiring growth hormone treatments endured daily injections that often led to nonadherence to therapy, due to pain and/or bruising with injection as well as fear of needles. The introduction of the Skytrofa Auto-Injector has reduced the frequency of injections to just once a week, while using a discreet needle.
The device previously received the PharmapackHealth Product Award for Patient-Centric Design in May 2022, and the Parenteral Drug Association (PDA) Innovation Award in October 2022.
How the Skytrofa Auto-Injector works
Skytrofa is a lyophilized powder available in a single-dose, dual-chamber, prefilled cartridge containing lonapegsomatropin-tcgd in one chamber and water in the other.
The cartridge is designed for subcutaneous injection once a week with the Skytrofa Auto-Injector. The delivery device provides a fully automated reconstitution of the lyophilized drug product, followed by a manual mixing step controlled by the device. The device automatically delivers the drug product after inserting the injection needle into the skin.
Built-in electronics and software assist the user during the entire preparation and injection sequence and confirm that the full dosage is delivered.
By making the device reusable and rechargeable, Phillips-Medisize and Ascendis Pharma have eliminated the waste of disposable injector pens and batteries with a full charge lasting four weeks, with one weekly injection. The life of the Auto-Injector is roughly four years or 210 injections.
John Christensen, senior project manager in contract development at Philips–Medisize, recently gave MD+DI a demonstration of how the Skytrofa Auto-Injector works.
Design considerations for the Skytrofa Auto-Injector
Paul Erik Fabricius, director of front-end innovation at Phillips-Medisize, said there were several key design considerations that went into this device because of the intended user population (parents of young children requiring growth hormone therapy).
The team wanted the design to be visually appealing for children and found that the white and bright green colors of the device made it more pleasing for this user population.
"We wanted to facilitate this very complex preparation procedure in a simple and safe manner," Fabricius told MD+DI. "That means both the physical handling of the device, for example it needs to stand up during the reconstitution to ensure that no drug is lost out through the needle, but you also need to have a nice grip when you do the injection."
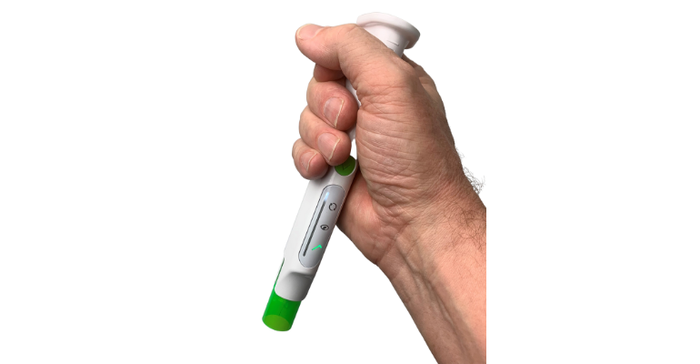
Phillips-Medisize conducted multiple usability studies with more than 300 participants that helped inform the design of the device. The participants included a mix of experienced and unexperienced users, trained and untrained users, healthcare providers and caregivers.
It was especially important to test the device with unexperienced, untrained users, Fabricius said, to ensure that it was as intuitive and simple to use as possible.
"That's a challenge because, if you imagine, when you see the device, you don't have any references because you've never seen a device like that," he said. "So, you start without any prior knowledge about how to use it, so basically anything can go wrong. But we've tested that with naive and untrained users, and they have all successfully received the dose."
Some of the users in these testing groups thought the device was actually needle-free because the 31-gauge needle is so thin, they didn't even feel it.

In addition to extensive usability studies, Fabricius said it was crucial to maintain an open dialogue with the customer throughout the design and development phase of the device.
"That was a key thing for success here because in the process things popped up where we needed to change things," he said. "For example, that manual mixing step John showed you, in the beginning we didn't think that was necessary. But it was simply due to the fact that we could see that the drug was not homogenous after the stable mixing step."
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
