Selecting the Proper Colorant for Thermoplastics
Medical Device & Diagnostic Industry MagazineMDDI Article IndexOriginally Published May 2000SPECIAL SECTIONTesting reveals which resins retain their physical properties when colored with zinc sulfide and titanium dioxide.
May 1, 2000
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
Originally Published May 2000
SPECIAL SECTION
Testing reveals which resins retain their physical properties when colored with zinc sulfide and titanium dioxide.
The color of a resin used in a medical device can be an important design consideration—for instance, color-coded arterial and venous probe cables on a blood-parameter monitor, to cite a recent example. Despite the existence of a good deal of information regarding colorant compatibility for various resin systems, little research has been reported on the subject of how colorants affect the physical properties of various engineering thermoplastics.1 This knowledge has generally been transmitted by word of mouth: an understanding of which colorants or additives are acceptable for a given resin system is typically passed from "generation to generation" within an organization's color department. Thus, either from prior structured testing of colorants, trial and error, or long-followed company practice, most colorists know what colorants to use for a particular resin system, but not the reasons behind the choice.
Anyone who specializes in one particular resin is likely to have a good understanding of the material's behavior when exposed to colorants. However, developing a broad enough knowledge base poses a problem for colorists or product designers who work with a wide range of commercially available resin families. If one takes into account the endless combinations of fillers, modifiers, reinforcements, and additives, acquiring comprehensive experience with colorants can be extremely difficult.
 Colorants can influence the tensile and impact strength as well as the viscosity of various engineering plastics. Photo courtesy of LNP Engineering Plastics Inc.
Colorants can influence the tensile and impact strength as well as the viscosity of various engineering plastics. Photo courtesy of LNP Engineering Plastics Inc.
What follows is an introductory review of the effects that two common colorants used in medical devices—titanium dioxide (TiO2) and zinc sulfide (ZnS)—have on the physical characteristics of various filled and unfilled amorphous and crystalline resin systems. The article will also attempt to explain the chemistry behind the observed property effects.
EXPERIMENTAL PROCEDURES
The colorants zinc sulfide and titanium dioxide were compounded with various engineering polymers on a 2½2-in., 30:1 single-screw extruder at levels up to 2.5% by weight. The resins employed included polycarbonate, polycarbonate with 20% glass fiber, polycarbonate with 10% carbon fiber, nylon 6/6, nylon 6/6 with 30% glass fiber, ABS, and polysulfone. The extruded materials were then tested for notched Izod impact strength, tensile strength, and melt viscosity.
As a control, the unfilled resins were tested "out of the bag" and after one extrusion pass. Conclusions regarding the effect of the colorants on a resin were made by observing any change (loss) in physical properties of a colored compound compared with the properties of the unfilled resin.
Although it has been shown that certain surface treatments on TiO2 can limit the degradation of physical properties in some resins, for this introductory study only one grade of TiO2 and ZnS extrusions were used for all the tests.2
RESULTS AND DISCUSSION
Unreinforced Nylon 6/6. Titanium dioxide, up to the 2.5% loading level, caused a slight decrease in Izod impact strength of unreinforced nylon 6/6. A steady decrease in melt viscosity was observed with increased TiO2 loadings.
Zinc sulfide yielded more-consistent results. Izod impact strengths and melt flow were barely affected at levels up to 2.5%. Melt viscosity decreased only slightly at a 2.5% loading.
Glass-Fiber-Reinforced Nylon 6/6. Upon the initial addition of TiO2 to 30% glass-fiber-reinforced nylon 6/6, Izod impact strength and tensile strength decreased considerably, but then remained at a constant level up to a 2.5% TiO2 loading. Melt viscosity decreased considerably with 0.5% TiO2, but then decreased at a slower rate up to the 2.5% level.

Figure 1. Effect of colorants on tensile strength of 30% glassfiber-reinforced nylon 6/6.

Figure 2. Effect of colorants on Izod impact strength of unreinforced polycarbonate.

Figure 3. Effect of colorants on viscosity (at 1000 1/sec) of unreinforced polycarbonate.
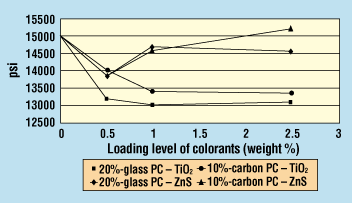
Figure 4. Effect of colorants on tensile strength of glass- or carbon-fiber-reinforced polycarbonate.
When ZnS was added to 30% glass-fiber-reinforced nylon 6/6, both Izod impact strength and tensile strength decreased considerably in response to low levels of the colorant, but subsequently increased as the level of ZnS was increased (Figure 1). At a 0.5% loading of ZnS, the melt viscosity decreased, but then remained constant when higher levels of ZnS were added.
Unreinforced Polycarbonate. There was no effect on the Izod impact strength of either the injection molding grade or lower-molecular-weight (fast-cycling) polycarbonate (PC) upon loadings of up to 1% of TiO2 (Figure 2).
Adding TiO2 to both grades of PC caused a slight decrease in melt viscosity with increasing levels of TiO2 (Figure 3).
The addition of up to 1.0% ZnS to the injection molding PC grade did not affect the resin's Izod impact strength. However, at 2.5% ZnS, there was an 84% decrease in strength. In the lower-viscosity grade, the effect was even more pronounced: an 87% reduction in strength at 1.0% addition, and complete failure at 2.5% (Figure 2).
Melt viscosity decreased slightly in the injection molding grade with up to 1.0% addition of ZnS. At 2.5% ZnS, melt viscosity decreased considerably (32%). In the lower-viscosity grade, the melt viscosity decreased 35% at 0.5% ZnS loading. As ZnS loading approached 2%, the viscosity had decreased to such an extent that it was no longer measurable (Figure 3).
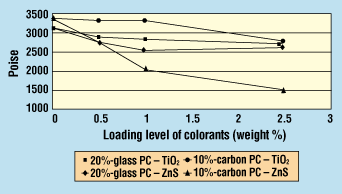
Figure 5. Effect of colorants on viscosity (at 1000 1/sec) of glass- or carbon-fiber-reinforced polycarbonate.

Figure 6. Effect of colorants on Izod impact strength of glass- or carbon-fiber-reinforced polycarbonate.

Figure 7. Effect of zinc sulfide on tensile strength of glass-fiber-reinforced polycarbonate.
Glass-Fiber-Reinforced Polycarbonate. For PC reinforced with 20% glass fiber, tensile strength dropped considerably with 0.5% TiO2, but remained constant at the 1.0% and 2.5% addition levels (Figure 4). Melt viscosity decreased only slightly with increasing TiO2 levels (Figure 5).
Izod impact strength dropped upon the addition of 0.5% TiO2 and continued to decrease up to the 1.0% loading. From 1.0% to 2.5% TiO2 addition, the Izod impact strength exhibited virtually no change (Figure 6).
The initial addition of ZnS (at 0.5%) caused a drop in tensile strength similar to that seen with TiO2 loading. However, as the level of ZnS increased, tensile strengths returned close to that of the unpigmented control sample (Figure 4). Izod impact strength with ZnS exhibited a similar effect: an initial drop in strength and then recovery at higher addition levels (Figure 6).
To investigate why tensile and Izod impact strengths would recover, additional samples of 10%- and 30%-glass-reinforced PC with varying amounts of ZnS were tested. These samples exhibited a similar pattern: after an initial drop in strengths at 0.5% ZnS, the tensile and Izod impact strengths did recover (Figures 7 and 8).
An examination of the melt viscosity data for the glass-fiber-reinforced PC colored with ZnS reveals that the melt viscosity drops upon increasing addition levels (Figure 9). This drop can explain why tensile and impact strengths are not affected, since the lower viscosity of the resin is actually "wetting out" the glass fibers more efficiently—resulting in better physical properties than is indicated by the data for unfilled PC.
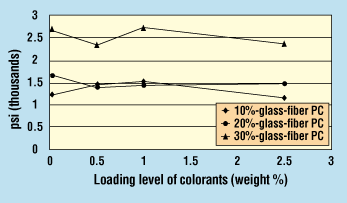
Figure 8. Effect of zinc sulfide on Izod impact strength of glass-fiber-reinforced polycarbonate.

Figure 9. Effect of zinc sulfide on viscosity (at 1000 1/sec) of glass-fiber-reinforced polycarbonate.
Carbon-Fiber-Reinforced Polycarbonate. Polycarbonate with 10% carbon fiber was evaluated to determine the effects the colorants would have on the physical properties of a resin that incorporated an alternative reinforcement.
Tensile strength for TiO2 decreased with increasing loading (Figure 4). Titanium dioxide addition did not affect the Izod impact strengths in the range tested (Figure 6), and melt viscosity values did not decrease until the 2.5% loading level (Figure 5).
With the ZnS colorant, tensile strength increased with higher loadings (Figure 4). There was a steady, slight decrease in Izod impact strength with increasing levels of ZnS (Figure 6). However, a dramatic decrease in melt viscosity occurred in the ZnS samples at 1.0% loading, and was even more pronounced at 2.5% (Figure 5).
Unfilled Polysulfone. There was a negligible effect on the Izod impact strength and melt viscosity of unfilled polysulfone at any TiO2 loading up to 2.5%.
Similarly, Izod impact strength and melt viscosity were not affected by up to 1.0% addition of ZnS. At 1.0% ZnS loading, however, Izod impact strengths dropped dramatically. Melt viscosity was not affected by further addition of zinc sulfide in the range tested.
Unfilled ABS. The Izod impact of unfilled ABS was barely affected by the addition of TiO2 in the range tested. There was a considerable increase in melt viscosity with increased TiO2 loading.
For ZnS, Izod impact strength steadily dropped to a loss of 30% at 2.5% loading. As with TiO2, there was a considerable increase in melt viscosity at a 0.5% level of ZnS—an increase that remained constant upon further loading.
CONCLUSION
From this investigation, it appears that titanium dioxide should be the colorant of choice for use with unfilled amorphous resins (polycarbonate, polysulfone, ABS), and zinc sulfide the colorant of choice for unfilled crystalline resins (nylon 6/6). An added benefit of using TiO2 is that its refractive index of 2.76 gives it 30—40% greater hiding power and tint strength than ZnS, which has a refractive index of 2.37.3
Our limited investigation of filled resin systems indicates that ZnS is preferable for glass-fiber-reinforced, crystalline resin systems because it is less abrasive than the rutile TiO2. (ZnS registers a 3 on the Mohs hardness scale, compared with 6–7 for TiO2.) For glass-fiber-reinforced polycarbonate, ZnS would seem to be the colorant of choice if the goal is physical property retention regardless of changes in melt viscosity. However, if a certain level of melt viscosity is to be maintained, TiO2 is the colorant to use. Melt viscosity test results led to the conclusion that the increases in physical properties with increases in ZnS loading are caused by the enhanced wettability of fibers resulting from the lowered viscosity of the polycarbonate. This enhanced wettability takes place because the ZnS acts as a Lewis acid, attacking the polycarbonate carbonyl groups and initiating degradation.4
For carbon-fiber-filled polycarbonate, the data indicate that TiO2 is clearly the colorant of choice since ZnS had a detrimental effect on Izod impact strength as the loading level increased whereas TiO2 exhibited little effect with increasing levels. Also, melt viscosity decreased considerably with the addition of more ZnS, but there was a negligible effect with the addition of TiO2.
ACKNOWLEDGMENTS
The authors would like to thank Maria Foss for her assistance during the preparation of this article.
REFERENCES
1. K Nangrani, R Wenger, and P Daugherty, "Effect of Pigments on the Flammability of Reinforced Thermoplastics," in Proceedings of the Society of Plastics Engineers 45th Annual Technical Conference (ANTEC 87) (Brookfield, CT: SPE, 1987), 15–18.
2. R Butler, "Limiting TiO2-Related Degradation in Engineering Thermoplastics," Plastics Compounding (November/December 1993).
3. T Webber, Coloring of Plastics (New York: Wiley, 1979), 38.
4. R Harris, Coloring Technology for Plastics (Norwich, NY: Plastics Design Library, 1999), 4.
Carl Bryce is product development chemist, Don Stengel is color technology manager, and Lori Tobin is senior laboratory technician at LNP Engineering Plastics Inc. (Thorndale, PA), which supplies a range of engineering polymers to the medical device industry.
Return to the MDDI May table of contents | Return to the MDDI home page
Copyright ©2000 Medical Device & Diagnostic Industry
You May Also Like


