News
Recall
Regulatory & Quality
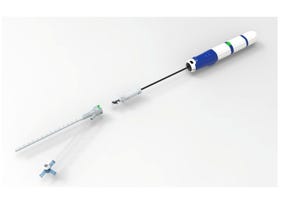
Elekta Disposable Biopsy Needle Recall Identified as Class IElekta Disposable Biopsy Needle Recall Identified as Class I
The company is recalling the needles from one batch as it may contain microscopic stainless-steel debris on the inside of the needle.
Sign up for the QMED & MD+DI Daily newsletter.

































.png?width=300&auto=webp&quality=80&disable=upscale)
