Graphic featuring MD+DI Senior Editor Amanda Pedersen and a logo designed for her weekly Pedersen's POV opinion column.
Diabetes
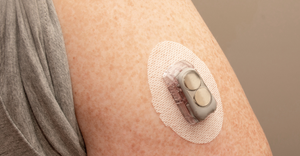
Help Me Convince My Husband to Try a CGMHelp Me Convince My Husband to Try a CGM
This week in Pedersen's POV, our senior editor turns to Dexcom for help convincing her husband to try continuous glucose monitoring (CGM).
Sign up for the QMED & MD+DI Daily newsletter.





.png?width=700&auto=webp&quality=80&disable=upscale)