Why 3-D Printed Skin Makes Sense
May 29, 2015
The beauty company L'Oreal is tapping the expertise of 3-D printed human tissue pioneer Organovo. We asked an additive manufacturing expert about the potential behind 3-D printing skin.
Chris Newmarker
|
Derek Mathers, business development manager at Worrell Design, thinks the prospect of 3-D printed skin could potentially have vast implications for healthcare. |
It is probably one of the most interesting 3-D printing stories so far this year: L'Oreal USA struck a partnership between its U.S.-based global technology incubator and tissue 3-D printing pioneer Organovo (San Diego). The idea is to leverage Organovo's proprietary NovoGen bioprinting platform and L'Oreal's expertise in skin engineering to develop 3-D printed skin tissue for potential products, as well as advanced research.
Organovo already has a track record in the space. With its NovoGen MMX bioprinting platform, Organovo has been focused on creating tissue samples that can be used by pharmaceutical companies in toxicology tests of new drug candidates. Organovo has successfully bioprinted lung, liver, skeletal muscle, cardiac, oncology (breast), blood vessel, bone, and nerve tissue. Its liver model is the furthest along, while its kidney model is next in line.
Qmed recently turned to Derek Mathers, business development manager at Worrell Design (Minneapolis), for some insights about the L'Oreal-Organovo partnership's potential.
Worrell itself has been annovator in the 3-D printing space, working with 3-D printing firm Stratasys to create a 3-D injection molding process that is meant to combine the benefits of the two manufacturing processes.
Here is what Mathers had to say about 3-D printed skin:
Qmed: What is 3-D printed skin, and what would it be good for?
Mathers: 3-D printing human skin tissue is currently administered in two forms: generic human "prototype" skin tissue that doesn't contain specific DNA information, and secondly in patient specific "end-use" skin tissue form that is printed directly onto a burn or skin wound.
The widespread adoption of generic 3-D-printed human skin tissue (as I call it "prototype tissue") will enable pharmaceutical, cosmetic, and medical companies to rapidly test the impact of their new devices and formulations with real human cells earlier in the development cycle, without the use of controversial human or animal testing. As the cost of printing human tissues goes down and the speed of this process increases, Organovo could be a critical player in accelerating and improving the development of drugs, lotions, and anything topical.
In order to maximize the benefits of 3-D printing--free complexity and customization, the ability to create architecture that isn't possible with other methods, and the ability to manufacture complete assemblies in one print--in any application is to produce end-use, functional parts. This is the driving force behind the industry tripling in five years to $4.1 billion (according to Wohlers 2015) in 2014.
This is why I am most excited about using 3-D printing to print patient-specific skin tissue back onto wounds, drastically improving the speed of recovery and quality of life of civilians and soldiers alike.
Qmed: I'd be interested to hear your thoughts on 3-D printed skin in general, and curious to know what the next step might be.
Mathers: From an immediate market-size perspective, L'Oreal spent $831 million in R&D in 2014. L'Oreal is a perfect example of a customer for the skin sample as-a-service offering.
Comparing this directly to a current customer of Organovo's liver sample service, biopharmaceutical firm Merck, for example, spent $6.5 billion in R&D in 2014 (nearly eight times as much), it appears that the liver service would have a more immediate return (depending on the frequency and amount of each print-as-a-service purchase). This would change drastically, however, if direct 3-D printing of patient-specific skin were to become a quick reality.
All of a sudden, Organovo's market could include the 486,000 U.S. citizens who require medical treatment for burn wounds annually (according to the American Burn Association). In addition, a military contract could be a very lucrative avenue for Organovo since nearly 20-30% of battlefield injuries are from burn wounds.
I spoke with the CEO of Organovo, Keith Murphy, last fall about the incredible things that Wake Forest School of Regenerative Medicine and the U.S. Army are doing with 3-D printing to repair burn wounds incurred by our soldiers--and what he said surprised me. Organovo is already there! They are working with Wake Forest to supply the printing platform for their model of:
Scanning a wound to determine the size and depth.
Taking a small sample of that patients stem cells.
Rapidly growing them throughout the day in a lab.
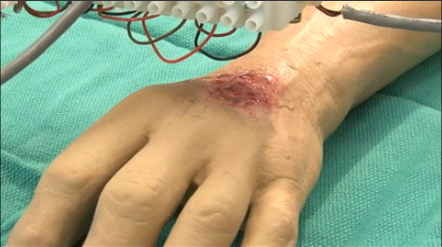
Printing-back multiple layers of skin shortly thereafter within a 10 x 10 cm swath of skin. This drastically reduces the amount of time it takes to recover from these wounds, removes the need for traditional skin grafts, and actually improves the scarring or permanent physical effects of the injury.
|
The Wake Forest School of Regenerative Medicine is exploring the use of 3-D printing to treat burns. |
In conclusion, even though the immediate profits from liver samples are important to pharmaceutical development, the likelihood of printing end-use functional livers seems further off than printing patient-specific skin onto wounds for rapid recovery. This is due to the difficulty of printing vascular structures into organs to supply those cells with nutrients.
So, if a consistent and effective method of 3-D printing of human skin onto wounds can be mastered and approved for our military, it will be more quickly be able to revolutionize care for civilians than printing everyone new livers. Although, humans will demand both in just a couple of years
Refresh your medical device industry knowledge at MD&M East in New York City, June 9-11, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)
